RESEARCH ARTICLE
Analyzing Effects of Multi-Wall Carbon Nanotubes (MWCNT) & Polyethylene Glycol (PEG) on Performance of Water Base Mud (WBM) in Shale Formation
Koorosh Tookalloo1, *, Javad Heidarian2, Mohammad Soleymani2, Alimorad Rashidi2, Mahdi Nazarisaram1
Article Information
Identifiers and Pagination:
Year: 2018Volume: 11
First Page: 29
Last Page: 47
Publisher Id: TOPEJ-11-29
DOI: 10.2174/1874834101811010029
Article History:
Received Date: 12/12/2017Revision Received Date: 12/02/2018
Acceptance Date: 18/03/2018
Electronic publication date: 30/04/2018
Collection year: 2018
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
Due to importance and unique properties of Multi-Wall Carbon Nanotube(s) (MWCNT), in the present study, effectiveness of these materials in Water Base Mud (WBM) is evaluated.
Objective:
The impacts of mud additives, local water and the addition of phases of bentonite and surfactants on the rheological properties, water loss and stability of water base mud in the absence of Multi-Wall Carbon Nanotube have been experimentally investigated.
Materials and Methods:
Then, the same experiment performed in the presence of Multi-Wall Carbon Nanotube to determine the efficiency and impact of Nanoparticles (NPs) on the properties of water base mud. The results have shown that additives, local water, Multi-Wall Carbon Nanotube dimensions, addition phase of bentonite and surfactants have influenced the rheological properties of the water base mud.
Results:
When Multi-Wall Carbon Nanotubes and polyethylene glycol alone or together are added, the performance terms of rheological properties decrease as by the subsequent order CNT; CNT + PEG; PEG. Multi-Wall Carbon Nanotube improves shale integrity and increases shale recovery.
Conclusion:
In general, the presence of Multi-Wall Carbon Nanotube increases the efficiency of polymers and rheological properties of the water base mud and eventually the shale stability is achieved.
1. INTRODUCTION
Nowadays, the maintenance of wellbore stability is very important aspect of drilling. Water invasion to the shale formations is resulted due to weakening of the wellbore and it causes many problems, e.g. stuck pipe and hole-collapse [1]. The problem of wellbore instability in the shale formation is well-known in the drilling industry, since over 75% of the drilled formations consist of shale rocks. Any problematic shale is a serious technical problem, which causes 90% of wellbore instability problems, time spending and revenue in the petroleum explorations [2].
Due to the characteristic of water sensitivity of the shale in relation to its ionic composition and clay content, the formation is seen as the most troublesome issue for the drillers. Shales are also troublesome, since they have a very low Nano-Darcy permeability with very small nanometer-sized pore throats that are not effectively sealed by the solids in conventional drilling fluids. The unstable tendency of the water sensitive shale may be related to water adsorption and clay hydration [3]
In the past, the balanced activity Oil Base Muds (OBM) were used for drilling through troublesome shale formations [4]. OBM subject to its superior shale stabilization characteristic can solve such problems of wellbore instability [5]. OBM is a key solution for maintaining stability of the shale [6]. However, the use of OBM is limited largely due to its environmental restriction (particularly in offshore drilling), costliness and safety [7].
Therefore, the design and development of water base mud (WBM) with OBM performance is currently seen as an area of great interest in the oil industry.
WBM, which is consisting potassium chloride (KCl) and polymer, was introduced in 1960s. Partially Hydrolyzed Polyacrylamide (PHPA) provides coatings and aids to stabilize the shale by polymer protective layer [6]. Van Oort by replacing OBM with WBM in some Algerian fields, proving that the application of specific additives, i.e. KCl and polymer improve shale stability [8]. In other areas, where the inhibition to restrain the chemical alteration of the shale is required, potassium-base muds may be used. Potassium ions exchange with sodium or calcium ions on the interlayered clays and smectites [5].
Conventional macro and micro base fluids (chemicals and polymers) have limited thermal stability. Moreover, they would get thermal degradation above 125-130 °C. Due to degradation, these chemicals cannot perform their desired function effectively in the drilling mud systems. Therefore, in order to reach the desired viscous and gelling properties under high pressures and high temperatures, the drilling mud must comprise of the specific components, e.g. Nanoparticles, which have stability under extreme conditions. Excellent thermal conductivity of Nanobase fluids associated with temperature and pressure tolerances, it can be a better choice . Nanoparticles have the potential to become a permanent constituent of all drilling mud systems, as they can be an efficient solution of many down-hole problems. NPs as a great alternative replace the traditional strategies and allow current drilling industry to go beyond the limits in order to reach those particular hydrocarbons regularly known as inaccessible [9].
WBM is rather more in contact with the clay than OBM. This contact may cause instability. In order to reduce the invasion of drilling fluids, the drilling fluids should form internal or external mud cake [10]. Normal additives cannot form good mud cake, because of small-sized pore throat and low shale permeability. Pore throat plugging may not be achieved in the shale, because of the large size of regularly used solid mud additives, which do not plug pore throat. Normal solid particles are approximately 100 times larger than pore throats [11]. Slow flux of filtrate of WBM into the formation leads to a significant pore pressure zone near the wellbore wall and subsequently wellbore instability. Therefore, the physical plugging of Nanoscale pore throats can be implemented to reach the several benefits, including pore pressure reduction owing to its feature of preventing the mud filtrate influx toward the shale, while the shale swelling is reduced owing to its feature avoiding the more interaction between the shale and mud filtrate, the high membrane efficiency is generated due to the shale permeability reduction and consequently the shale stability [8].
The experiments have shown that NPs improve the rheological properties of Nanofluids [12]. Recently, Nanobase drilling fluids have been formulated and resulted in the improvement of rheological properties, i.e. the stability and the gelling property, and the ultrathin mud cake. Nanobase fluids reduce any damage during the formation by the elimination of spurt loss. Ultrathin mud cake dramatically decreases the differential pipe sticking and, as a result, Nanobase fluid is applied in the formation with the high permeability [13].
Due to the following facts, the Nano additives (Nanos) have the potential to be used in the drilling operations; firstly, the huge surface area of NPs increases the interactions between Nanos and the reactive shale and resolve borehole problems. Secondly, the less kinetic energy of NPs reduces the abrasive effect of Nanos on down-hole equipment, which leads to damaging. In conclusion, Nanos are very effective at low concentrations, as it is an advantage for the ecosystem and industry [14]. An alternate approach for shale stability base on the research works of Sensory, Chenevert and Sharma conducted on nano-silica (nS), it is shown that the flow of fluids through an Atoka shale plug could be shut off by NPs. In fact, what they essentially conducted was plugging the nano-sized pores in the shale by using the nano-silica particles [10]. Previously, the test results on the gas shale samples shown that formulating the mud properly and choosing appropriate nano materials with proper size and concentration are the keys to prevent water flow into the shale samples with a wide range of initial permeability in the range of 1 to 100.000 Nano-Darcy (nD) [14].
Adding the functionalized Multi-Wall Carbon Nanotube (MWCNT) increases the yield point and plastic viscosity of WBM, reduces spurt lost, constructs uniform mud cake, as the pipe sticking risk is reduced and torque decreases during the drilling operations drag. By using MWCNT’s enhance the annular viscosity, therefore hole-cleaning and lifting capacity compare with conventional WBM is improved [15]. MWCNT under high temperature and pressure wells can be replaced the conventional viscosity agents, e.g. organic polymers, clay or fatty acids. The application of MWCNT with the specific trace amounts, preferably less than 3% by weight, would not cause additional problems, so the mud can pump better [16]. Drilling fluid containing graphene-base materials reduces the shale permeability, closing its pores and achieving better results than the conventional polymers, particularly at high temperatures [17]. MWCNT with the shield agent (PHPA and PAC) allows us to use fewer NPs, which produces no spurt during API filtration test [3]. MWCNT improves the rheological properties, increasing the thermal conductivity and the shear stress, while water loss is reduced [18], the corrosion resistance is improved, the speed rate is increased, as the electrical conductivity, wettability alteration, and the sustainability of the shale are improved. Quintero et al. found that the concentrations of NPs for the shale stability should be in the range of 5 to 150,000 ppm. They found that NPs with surface charge such as MWCNT could aid to reach the shale stability. The small size of NPs allows good access to the shale matrix [19]. Amanullah et al. found that MWCNTs may increase the viscosity of WBM fluid composition by at least 10 centipoise (cp), providing additional gelling capacity, whereas the water loss and the thickness of mud cake are reduced [20].
The present study investigates the effect of MWCNT and PEG on the rheological properties, the shale recovery and the shale integrity of WBM.
2. MATERIALS AND RESEARCH METHODOLOGY
2.1. Materials
Bentonite, potassium chloride, Xanthan Gum (XG) (viscosity agent) and PHPA were purchased from Kimyagaran Drilling Fluids, a leading trading company in the production and supply of the raw materials for oil and gas drilling mud in Iran. Caustic Soda and polyethylene Glycol 600 were purchased from Merck in Iran. Low viscosity grade of polyanionic cellulose (fluid loss control additive) was purchased from Henzak Chemie Company in Iran. MWCNT NO.1 (MWCNT, diameter: 10-20 nm, purity > 95%, length of 10 μm and surface area: 250 m2/gr) and MWCNT NO.2 (MWCNT, diameter ≥ 50 nm, purity > 95%, length of 20-30 μm) produced in Research Institute of Petroleum Industry of Iran (RIPI). Surfactants used in this research are Sodium Dodecyl Sulfate (SDS), Tween 80 (T80) and Gum Arabic (GA) that are commonly used in the oil industry.
The local water analysis was done by ASTMD4691 method and the results are shown in Table 1.
| No. | Component | Amount | Unit |
|---|---|---|---|
| 1 | Na | 1.0 | g/L |
| 2 | Mg | 0.15 | |
| 3 | Ca | 0.45 | |
| 4 | K | 9 | mg/L |
| Sample | Test Method | Result |
|---|---|---|
| D3536.75 | XRD | 1-Quartz, 2-Kaolinite, 3-Feldspar |
2.2. Experimental Procedure
2.2.1. Samples Preparation
The entire tests were conducted in the Research Institute of Petroleum Industry (RIPI) of Iran.
First phase: Preparing the Nanofluid sample consisting of NPs, surfactants and water in their formulation. Nanofluids were prepared according to the formulation mentioned in Table 3.
| Additive | Unit | Amount |
|---|---|---|
| Water | ml | 20 |
| Surfactant | gr | 0.35 |
| MWCNT | gr | 0.35 |
In first phase, surfactants were weighed and added into the beaker containing 20 cc water and stirred by a magnetic stirrer for 5 minutes to prepare the target unique fluid. Afterward, the weighed MWCNT was added to the sample. Formulation of Nanofluid is given in Table 3.
As shown in Fig. (1), after adding MWCNT into the sample containing water and surfactants, MWCNT became sediment. The ultrasonic bath is used for dispersing MWCNT in the sample.
 |
Fig. (1). MWCNT does not disperse in sample. |
Second phase: Ultrasonication
The sample was applied by ultrasound in the ultrasonic bath (P 120 H, Elmasonic Co., Germany) for 30 minutes under a frequency of 37 kHz, 100 W and ambient temperature. In Fig. (2), the Nanofluid sample after ultrasonication is shown. This figure shows that MWCNT is completely dispersed in the sample.
 |
Fig. (2). The Nanofluid sample after ultrasonication. |
In Table 4, the formulation of Nanofluids with different surfactants is illustrated.
| NO. | Sample | CNT type | CNT (gr) | Distilled water (ml) | Local water (ml) | SDS (gr) | GA (gr) | T80 (gr) |
|---|---|---|---|---|---|---|---|---|
| 1 | Nano DF | NO.1 | 0.35 | 20 | - | 0.35 | - | - |
| 2 | Nano DF/RPF | NO.1 | 0.35 | - | 20 | 0.35 | - | - |
| 3 | Nano DF/RPG | NO.1 | 0.35 | - | 20 | - | 0.35 | - |
| 4 | Nano DF/RPT | NO.1 | 0.35 | - | 20 | - | - | 0.35 |
| 5 | Nano DF/RPGBF | NO.1 | 0.35 | - | 20 | - | 0.35 | - |
| 6 | Nano DF/RPG.GEF | NO.1 | 0.35 | - | 20 | - | 0.35 | - |
| 7 | Nano DF/RPGF | NO.1 | 0.35 | - | 20 | - | 0.35 | - |
| 8 | Nano DF/RMG.GEF | NO.1 & NO.2 | 0.175 & 0.175 | - | 20 | - | 0.35 | - |
Third phase: Preparing base mud (sample without NPs)
In Table 5, the base mud with different formulations and Mixing Time (MT) durations are represented.
| NO. | Sample | Distilled water (ml) | local water (ml) | Bentonite (g/350ml) | KCl (g/350ml) | NaOH (g/350ml) | XG (g/350ml) | PAC (g/350ml) | PHPA (g/350ml) | PEG (g/350ml) | rpm |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MT:10 min | MT:3 min | MT:2 min | MT:5 min | MT:5 min | MT:10 min | MT:5 min | |||||
| 1 | DF | 340 | - | 10 | 11.3 | 0.3 | 0.6 | 2 | 1 | - | 3000 |
| 2 | Nano DF | 320 | - | 10 | 11.3 | 0.3 | 0.6 | 2 | 1 | - | 3000 |
| 3 | DF/BF | - | 340 | 10 | 11.3 | 0.3 | 0.6 | 2 | 1 | - | 3000 |
| 4 | Nano DF/RPF | 320 | 10 | 11.3 | 0.3 | 0.6 | 2 | 1 | - | 3000 | |
| 5 | Nano DF/RPG | - | 320 | 10 | 11.3 | 0.3 | 0.6 | 2 | 1 | - | 6000 |
| 6 | Nano DF/RPT | - | 320 | 10 | 11.3 | 0.3 | 0.6 | 2 | 1 | - | 6000 |
| 7 | DF/BFBF | - | 340 | 10 | 11.3 | 0.3 | 0.6 | 2 | 1 | - | 6000 |
| 8 | Nano DF/RPGBF | - | 320 | 10 | 11.3 | 0.3 | 0.6 | 2 | 1 | - | 6000 |
| 9 | DF/BF1B | - | 340 | 10 | 11.3 | 0.3 | 0.6 | 2 | 1 | 0.75 | 6000 |
| 10 | Nano DF/RPG.GEF | - | 320 | 10 | 11.3 | 0.3 | 0.6 | 2 | 1 | 0.75 | 6000 |
| 11 | DF/BFGEF | - | 340 | 10 | 11.3 | 0.3 | 0.6 | 2 | 1 | 0.75 | 6000 |
| 12 | Nano DF/RPGF | - | 320 | 10 | 11.3 | 0.3 | 0.6 | 2 | 1 | 6000 | |
| 13 | Nano DF/RMG.GEF | - | 320 | 10 | 11.3 | 0.3 | 0.6 | 2 | 1 | 0.75 | 6000 |
Firstly, the drilling fluid additives were weighted and mixed with water in Hamilton Beach Stirrer with the same order of the additive phases and the mixing time mentioned in Table 5. Finally, Nanofluids, produced in the first phase, were added and mixed for 10 minutes with the base mud sample. For preparing the base mud 340 ml water was used and, for Nanofluid, 320 ml water (distilled or local water) was used.
2.2.2. Measuring Rheological Properties of WBM
Rheological properties of samples measured by Fann Viscometer, Model 35SA, under six differences rpm (600, 300, 200, 100, 6 and 3 rpm) to reach plastic viscosity and yield point of the samples. PH of the samples was measured by pH meter, and then the mud density was measured by the mud balance. At last, API Filter Press Apparatus was used to measure the filtration properties of the samples.
2.2.3. Shale Recovery Test
The procedure of modified API RP 13-I was used in this study. Firstly, the shale grounding to a particle size less than 4 mm (Mesh No.5) and larger than 2 mm (mesh No. 10) was applied.
20 gr shale was added to the fluid samples. The samples allowed rolling in a roller oven for 8 hours at 121 °C (250 °F) for hot rolling. Then, the samples screened through mesh NO. 35 screen (0.5 mm) and then washed by using a saturated aqueous solution of sodium chloride and potassium chloride brine (15%). The remaining shale on the sieve was washed to remove the mud stuck to shale particles, before drying and reweighing. The high weight sample passed the screen (Mesh 35) is an indication of the shale instability.
In Eq. 1, the shale recovery is calculated as:
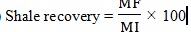 |
(1) |
MI = Initial mass of shale sample
MF = Final dry mass of shale sample
2.2.4. Shale Integrity Test
A shale tablet with a thickness diameter of 1 inch and 1 cm was put into 350 cc of WBM sample. Shale tablet was placed for 24 hours at temperature of 93 °C (200 °F), and then shale tablet was put to dry in room temperature. Another shale tablet was put in the OBM sample and compared together physically, after 2 tablets were dried.
3. RESULT AND DISCUSSION
3.1. Rheological Properties
In Table (6) the data obtained from different WBMs is illustrated. The rheological and filtration properties of the muds are presented in Table 7.
| No. | Sample | Ө 600 (rpm) |
Ө 300 (rpm) |
Ө 200 (rpm) |
Ө 100 (rpm) |
Ө 6 (rpm) |
Ө 3 (rpm) |
|---|---|---|---|---|---|---|---|
| 1 | DF | 49 | 36 | 30 | 23 | 11 | 9 |
| 2 | Nano DF | 81 | 60 | 51 | 40 | 17 | 14 |
| 3 | DF/BF | 51 | 40 | 34 | 27 | 13 | 10 |
| 4 | Nano DF/RPF | 65 | 48 | 40 | 31 | 15 | 12 |
| 5 | Nano DF/RPG | 53 | 41 | 35 | 28 | 13 | 11 |
| 6 | Nano DF/RPT | 50 | 37 | 31 | 25 | 12 | 10 |
| 7 | DF/BFBF | 51 | 39 | 34 | 27 | 14 | 12 |
| 8 | Nano DF/RPGBF | 48 | 37 | 32 | 26 | 13 | 11 |
| 9 | DF/BF1B | 36 | 26 | 22 | 17 | 8 | 6 |
| 10 | Nano DF/RPG.GEF | 49 | 38 | 33 | 26 | 13 | 11 |
| 11 | DF/BFGEF | 64 | 48 | 42 | 34 | 21 | 15 |
| 12 | Nano DF/RPGF | 54 | 42 | 36 | 29 | 14 | 12 |
| 13 | Nano DF/RMG.GEF | 52 | 40 | 36 | 29 | 16 | 13 |
In Fig. (3), the amount of filtrate loss and mud cake thickness of Nano DF/RPG is shown.
In Fig. (4), the samples frothed in the presence of PEG are shown. Suitable rheological properties of drilling mud for shale stability are shown in Table 8 [21]. Plastic viscosity should be in the range of 20 to 25 cP, the yield point should be in the range of 15 to 20 Pa, Ө3 should be at the range of 0 to 5 and the fluid loss is less than 5 cc. The data is obtained from the viscometer for the shale stability as represented in Table 8 [21]. In comparison of the results of Tables (6 and 8), it is shown that the data obtained from our present research are almost an acceptable and consistent range for the shale stability.
 |
Fig. (3). Amount of filtrate loss and mud cake thickness of Nano DF/RPG. |
 |
Fig. (4). (A) Nano DF/RPG.GEF frothed after mixing; (B) DF/BF1B has stable froth after mixing. |
| NO. | Sample | PV (cp) | YP | pH | FL (cc) | CT (mm) | Density |
|---|---|---|---|---|---|---|---|
| 1 | DF | 13 | 23 | 11.2 | 9 | 0.13 | - |
| 2 | Nano DF | 21 | 39 | 9.9 | 7.5 | 0.35 | - |
| 3 | DF/BF | 11 | 29 | 11 | 7.6 | 0.5 | - |
| 4 | Nano DF/RPF | 17 | 31 | 9.7 | 9 | 0.4 | - |
| 5 | Nano DF/RPG | 12 | 29 | 9.8 | 8 | 0.83 | - |
| 6 | Nano DF/RPT | 13 | 24 | 9.9 | 8.7 | 0.68 | - |
| 7 | DF/BFBF | 12 | 27 | - | 7.1 | 0.72 | 65 |
| 8 | Nano DF/RPGBF | 11 | 26 | - | - | - | 65 |
| 9 | DF/BF1B | 10 | 16 | - | 9.2 | 0.75 | 65 |
| 10 | Nano DF/RPG.GEF | 11 | 27 | 9.8 | 6.4 | 0.91 | 63 |
| 11 | DF/BFGEF | 16 | 32 | 11.3 | - | - | - |
| 12 | Nano DF/RPGF | 12 | 30 | 10.3 | - | - | - |
| 13 | Nano DF/RMG.GEF | 12 | 28 | 12 | - | - | - |
| Ө600 | Ө300 | Ө200 | Ө100 | Ө6 | Ө3 |
|---|---|---|---|---|---|
| Less than 90 | Less than 65 | Less than 60 | Less than 50 | Less than 10 | Less than 5 |
In Fig. (5), the related data of Table (6) is illustrated.
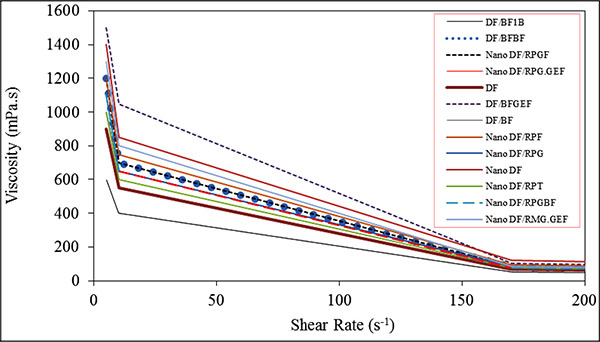 |
Fig. (5). Viscosity-shear rate curve below 200 s-1. |
The viscosity rates of different samples at shear rates higher than 170 s-1 are almost identical. Therefore, we compare viscosity with shear rate below 200 s-1 and the data is illustrated in Fig. (5).
3.1.1. Effect of Distilled Water and Local Water on Rheological Properties of WBM
Fig. (6) shows the effect of water on the mud viscosity. As illustrated in Table (5), DF/BF and Nano DF/RPF have been prepared with local water; however, Nano DF and DF have been made of distilled water. In shear rate, below 200 s-1 viscosity of DF/BF is greater than DF, which is prepared with distilled water, while the viscosity of Nano DF/RPF is less than Nano DF.
According to Fig. (6), the apparent viscosity of Nano DF and DF samples is more than samples prepared by the local water. DF yield point is less than DF/BF and the yield point of Nano DF/RPF is less than Nano DF. In other words, the local water reduces the apparent viscosity and the yield point of the samples. The local water reduces the amount of water loss and increases mud cake thickness in the base mud (DF and DF/BF). By comparing the samples of Nano DF/RPF and DF/BF, it can be seen that MWCNT reduces the mud cake thickness and increases the water loss rate. In general, the performance of MWCNT in the presence of salt (local water) is less than distilled water.
The local water increases the viscosity of base mud, however, by adding MWCNT to the base mud, it is the viscosity decreases. The reason is that there are many polar minerals in the local water and MWCNT is non-polar, therefore, MWCNT cannot absorb to the polar minerals of the local water and the uniform fluid is not achieved. Meanwhile, in the base mud, all additives (polymers) are polar and good absorption with salts was found in the local water, therefore a homogeneous fluid is generated and the viscosity of the base mud is increased.
 |
Fig. (6). Effect of distilled water and local water on rheological properties of WBM. |
3.1.2. Effect of Bentonite Addition Phase on Rheological Properties of WBM
In Fig. (7), the effect of bentonite addition phase on the rheological properties of Nanofluid is shown. Additives and their concentration in Nanofluid are the same in Fig. (7), however, the difference is seen in bentonite addition phase. In the Nano DF/RPGF, bentonite is added into the mud, before KCL and NaOH, while in the Nano DF/RPGBF, bentonite is added to the sample immediately, after the NaOH; and finally in the sample of Nano DF/RPG bentonite is added into the sample at last.
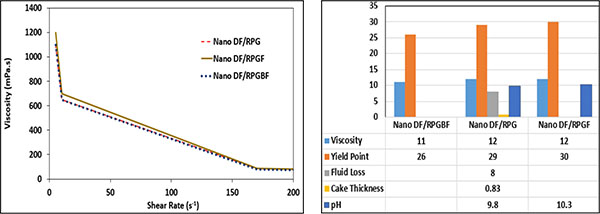 |
Fig. (7). Effect of bentonite addition phase on rheological properties of Nanofluid. |
Since bentonite is formed from the fine particles of the clay, so when it is added to the sample before KCl, its saturation will be better in the sample and its viscosity will be increased, however, when it is added, after KCl into the sample, KCl prevents bentonite from swelling and distribution in the sample and ultimately viscosity will be decreased. As it is shown in Fig. (7), Nano DF/RPGF has higher viscosity than Nano DF/RPGBF and Nano DF/RPG.
In Fig. (8), the effect of the bentonite addition phase on the rheological properties of the base mud is shown. DF/BFGEF and DF/BF1B include PEG in their formulation. In DF/BFGEF, bentonite is added to the sample before KCl and, in DF/BF1B, bentonite is added to the sample after NaOH.
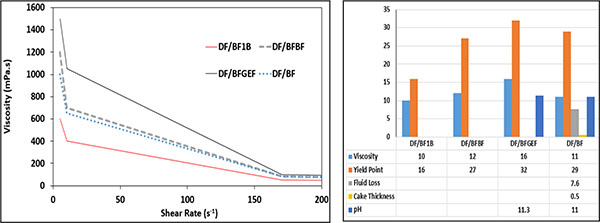 |
Fig. (8). Effect of bentonite addition phase on the rheological properties of base mud. |
If bentonite is added to the sample before KCl in the shear rate below 200 s-1, the viscosity will be increased. According to Fig. (8), the plastic viscosity and yield point of DF/BFGEF are comparatively higher than DF/BF1B. In DF/BFBF, bentonite is added to the sample before KCl, therefore it is better scattered in the sample and has higher viscosity compared to DF/BF. Bentonite is a type of clay and KCl prevents it from swelling, so when bentonite is added to the sample before KCl, drilling fluid rheology properties will be more affected.
3.1.3. Effects of Surfactants on Rheological Properties of WBM
In Fig. (9), the effects of surfactant on the rheological properties of WBM is shown. In this section, the effect of different surfactants will be compared on the viscosity of WBM. SDS surfactant is used for preparing Nano DF/RPF, GA Surfactant is used for preparing Nano DF/RPG and T80 Surfactant is used for preparing Nano DF/RPT. Other additives are the same in all three samples. As shown in Fig. (9), SDS increases the plastic viscosity, the yield point, and the water loss of Nano DF/RPF more than the other surfactants and decreases the mud thickness. The problem of SDS is that it generates stable froth in the samples as shown in Fig. (10). GA distributes MWCNT in the mud homogeneously, where it does not produce froth after stirring and produces stable WBM. GA in comparison with T80 increases the yield point and decreases the water loss. T80 is the same as SDS, where it produces stable froth during stirring. Thus, in the production of other samples GA was used as the surfactant.
 |
Fig. (9). Effects of surfactants on rheological properties of WBM. |
 |
Fig. (10). Sample froths due to SDS surfactant. |
It is notable that after adding Nanofluid, prepared by SDS surfactant, to the base mud sample and mixing it, as shown in Fig. (10), the sample strongly frothed leading to lower mud weight thus affecting the rheological properties. Although to some extent SDS improves the rheological properties of the fluid samples, in most of the tests performed in the present research, GA is used as the surfactant.
As shown in Fig. (11), SDS surfactant has a polar (hydrophilic) head and a nonpolar (hydrophobic) head. The polar head generates a bond with the base mud and the nonpolar head generates a bond with MWCNT, followed by increased viscosity rate and improved rheology properties of the mud.
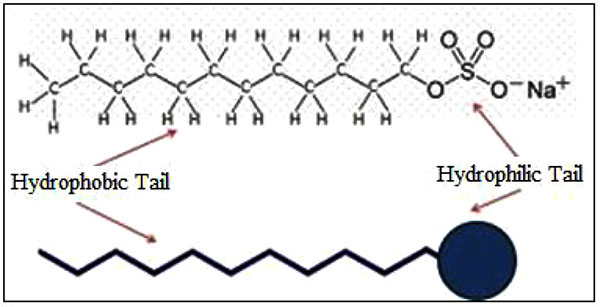 |
Fig. (11). SDS surfactant. |
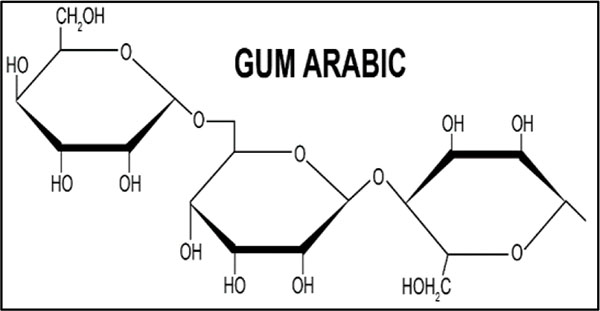 |
Fig. (12). GA surfactant. |
As shown in Fig. (12), GA and base mud are polar and MWCNT is nonpolar, thus not generating a good bond between MWCNT, GA and the base mud. It is the reason that there is a lower viscosity of the sample with GA compared to the sample generated by SDS.
T80, as illustrated in Fig. (13), has a polar and a nonpolar head, however, its polar head is mainly composed of ether groups. The ether polarity is low, therefore it generates a weak bond with the mud. It is the reason that Nano DF/RPT has lower viscosity and less improvement in the rheological properties than other samples.
 |
Fig. (13). Tween 80 surfactant. |
GA is a relatively high molecular weight polymer, T80 is a low molecular weight polymer and SDS is a simple molecule. Polymers have higher molecular weight and form a film to cover the pores of the filter paper, thereby reducing water loss. In Fig. (9), the quantity of water loss in the samples made by GA is lower than other samples.
3.1.4. Effect of Polyethylene Glycol (PEG) on Rheological Properties of WBM
In Fig. (14), the effect of PEG on the rheological properties of the WBM is shown. DF/BF1B and Nano DF/RPG.GEF consist of PEG in their formulation, however, Nano DF/RPGF and DF/BFBF does not have PEG in their formulation. As shown in Fig. (14), PEG reduces the viscosity and the yield point of the samples.
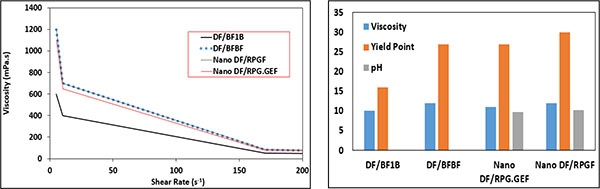 |
Fig. (14). Effects of polyethylene glycol on rheological properties of WBM. |
In Fig. (15), Polyethylene glycol structure is shown.
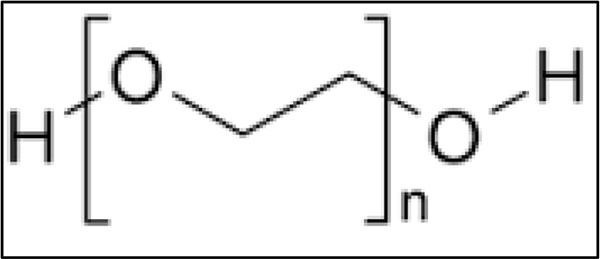 |
Fig. (15). Polyethylene glycol. |
By comparing the two samples of Nano DF/RPG.GEF and Nano DF/RPGF, it is obvious that MWCNT (nonpolar) forms a nonhomogeneous sample; whereas a more non-homogenized sample is formed, when PEG (nonpolar) is added. Thus, the viscosity of the sample containing MWCNT and PEG is less than the sample without PEG. In both samples, bentonite is added before KCl. As shown in Fig. (14), when PEG is added, the viscosity of the sample consisting of MWCNT is reduced.
3.1.5. Effects of Nanoparticles on Rheological Properties of WBM
Nano-carbon particles are added to the base mud to determine the effects of MWCNT on the performance of WBM. In Table (9), the Nano-mud and equivalent base mud with the same additives and concentrations are shown.
| NO. | Nano Mud | Equivalent Base Mud |
|---|---|---|
| 1 | Nano DF | DF |
| 2 | Nano DF/RPF | DF/BF |
| 3 | Nano DF/RPG.GEF | DF/BFGEF |
| 4 | Nano DF/RPGBF | DF/BFBF |
In Fig. (16), the effects of Nanoparticles on the rheological properties of WBM are shown. As it is evident in Fig. (16), by adding MWCNT to the base mud, it leads to increased viscosity and yield point of WBM. By comparing DF/BF and Nano DF/RPF, it is seen that Nanoparticles can reduce the thickness of the mud cake, however, the quantity of water loss is increased. By adding MWCNT to DF sample (Nano DF), the plastic viscosity and yield point is increased and water loss of DF is reduced.
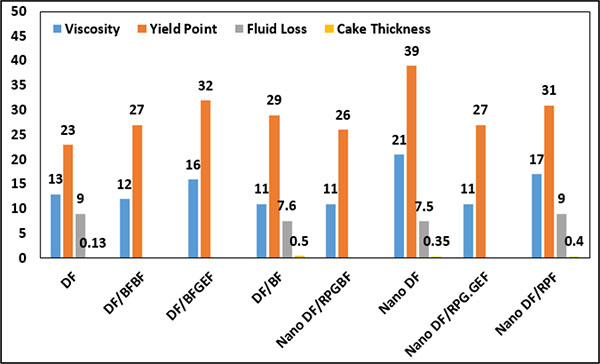 |
Fig. (16). Effects of Nanoparticles on rheological properties of WBM. |
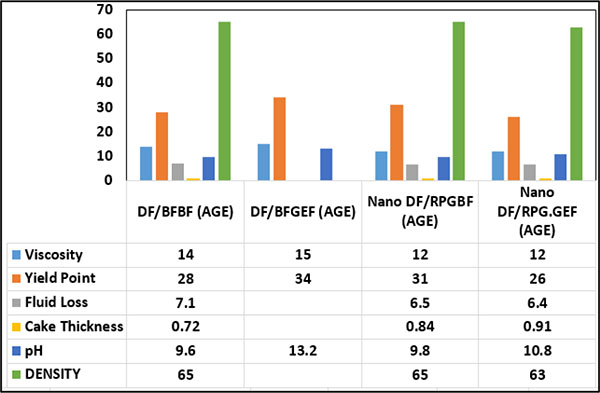 |
Fig. (17). Effect of Nanoparticles on rheological properties of WBM after 16 hours aging. |
In Fig. (17), the effects of Nanoparticles on the rheological properties of the WBM after 16 hours aging is shown. As shown in Fig. (17), after 16 hours by comparing the cases of DF/BFBF and Nano DF/RPGBF, it can be seen that MWCNT increases the thickness of the mud cake and reduces water loss.
CNT has a high modulus and high surface area and thus improves the rheological properties of mud. The addition of MWCNT reduces the plastic viscosity and yield point of DF/BFGEF sample due to the presence of PEG.
By adding MWCNT to the base mud without PEG, it improves the rheological properties of the mud, as the reasons are mentioned earlier.
3.1.6. Effects of MWCNT Dimensions on Rheological Properties of WBM
In Fig. (18), the effects of MWCNT dimensions on the rheological properties of WBM are shown. The diameter and length of MWCNT used in Nano DF/RMG.GEF is more than MWCNT used in Nano DF/RPG.GEF. Due to the higher modules and strengths of larger MWCNT compared to the smaller ones, the plastic viscosity, yield point and pH of Nano DF/RMG.GEF are higher than Nano DF/RPG.GEF.
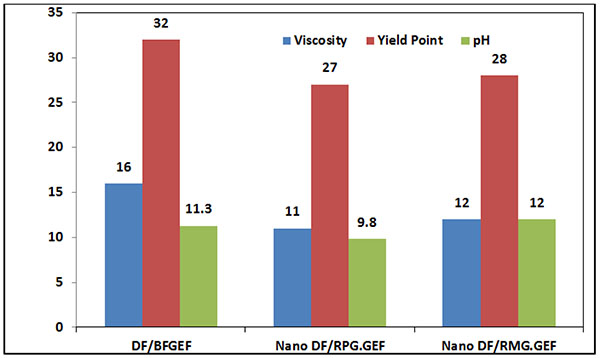 |
Fig. (18). Effect of MWCNT dimensions on rheological properties of WBM. |
3.1.7. Role of MWCNT on Aging of WBM
In Table (10), the rheological properties of some samples after 16 hours aging is shown. In this part, the role of aging on the rheological properties of WBMs is examined. For this purpose, the rheological properties were measured immediately after preparing the sample and once after 16 hours. It is very important that the rheological properties of WBM sample does not alter over time and it is stable. If the mud properties are affected excessively over time, it causes huge losses in the drilling industries. For this purpose, the drilling fluids should have maximum stability during the drilling operations.
| NO. | Sample (age) | PV (cp) | YP | pH | FL (cc) | CT (mm) | Ө600 | Ө300 | Ө200 | Ө100 | Ө6 | Ө3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | DF/BFBF | 14 | 28 | 9.6 | - | - | 56 | 42 | 36 | 28 | 15 | 13 |
| 2 | DF/BF1B | 11 | 19 | 9.6 | - | - | 41 | 30 | 25 | 19 | 8 | 6 |
| 3 | DF/BFGEF | 15 | 34 | 13.2 | - | - | 64 | 49 | 43 | 35 | 17 | 14 |
| 4 | Nano DF/RPGF | 12 | 13 | 11.4 | - | - | 54 | 42 | 36 | 29 | 14 | 12 |
| 5 | Nano DF/RPG.GEF | 12 | 26 | 10.8 | - | - | 50 | 38 | 33 | 26 | 13 | 11 |
| 6 | Nano DF/ RPGBF | 12 | 31 | 9.8 | 6.5 | 0.84 | 55 | 43 | 37 | 29 | 15 | 13 |
| 7 | Nano DF/RMG.GEF | 13 | 31 | 12 | 7 | 0.55 | 57 | 44 | 37 | 33 | 15 | 13 |
In Fig. (19), the viscosity versus shear rate after 16 hours aging is shown.
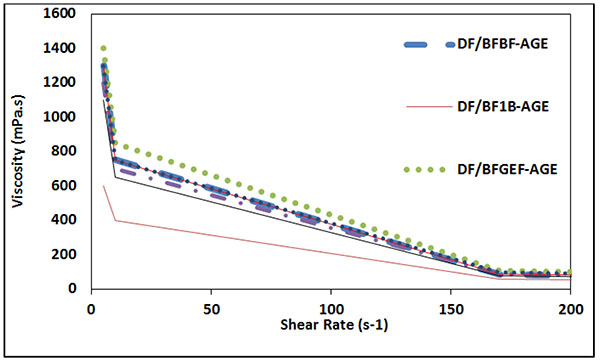 |
Fig. (19). Viscosity versus shear rate after 16 hours aging. |
In Fig. (20), the plastic viscosity of the mud samples after 16 hours aging is compared.
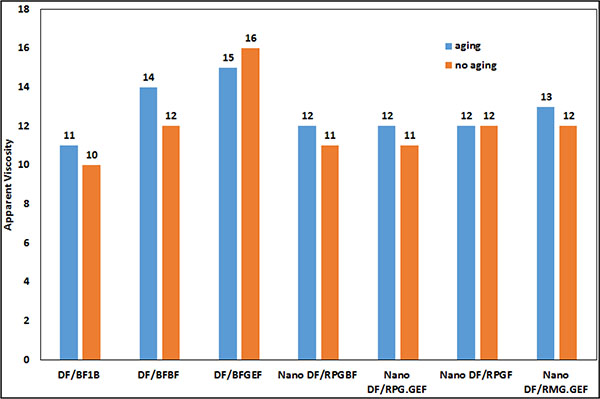 |
Fig. (20). Comparison of apparent viscosity of mud samples after 16 hours aging. |
In Fig. (21), the yield point of the mud samples after 16 hours aging is compared.
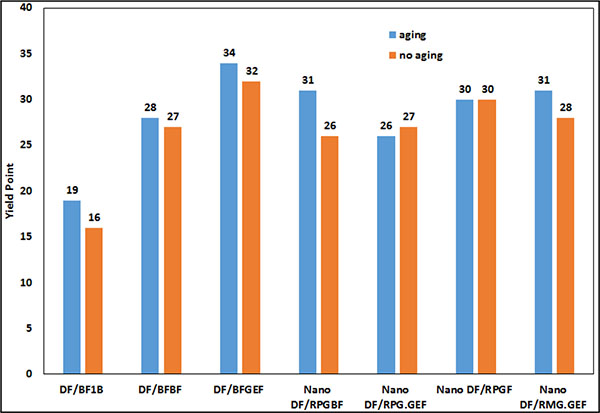 |
Fig. (21). Compare yield point of mud samples after 16 hours. |
AS shown in Figs. (20 and 21), the properties of the samples having MWCNT did not change excessively after 16 hours aging and their plastic viscosity and yield point are almost stable. The reason is that MWCNT can maintain the polymers against degradation as mentioned by Halali [22].
By observation of the samples shown in Fig. (22), it seems that the samples have good stability after 15 days.
 |
Fig. (22). (A) Nano DF/RPT is still stable after 15 days; (B) Nano DF/RPG is still stable after 15 days. |
According to Tables (7 and 10) and by comparing the both samples of Nano DF/RPF and Nano DF/RPGBF, we observed that MWCNT increases the thickness of the mud cake and reduces water loss after 16 hours.
3.2. Shale’s Recovery Test Results
In Fig. (23), the quantities of the shale recovery of the samples are shown. The data obtained from shale recovery tests are represented in Table (11). As shown in Fig. (23), by Nano DF/RPGF and DF/BFGEF, it is determined that MWCNT in comparison with PEG reduces the shale recovery.
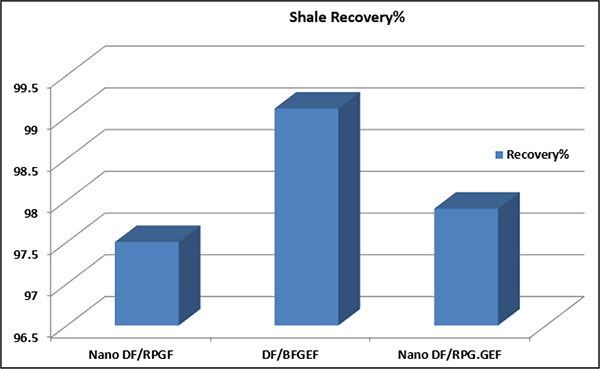 |
Fig. (23). Quantities of shale recovery of the samples. |
| No. | Final Dry Mass of Shale Sample | Initial Mass of Shale Sample | Sample | Shale Recovery % |
|---|---|---|---|---|
| 1 | 19.5 | 20 | Nano DF/RPGF | 97.5 |
| 2 | 19.82 | 20 | DF/BFGEF | 99.1 |
| 3 | 19.58 | 20 | Nano DF/RPG.GEF | 97.9 |
When PEG is absorbed on the surface of the shale, it maintains the shale particles together and improves the shale recovery of the samples. By comparing the samples of Nano DF/RPG.GEF and DF/BFGEF, it can be seen that by adding MWCNT reduces the shale recovery subject to the absorption some quantity of PEG by MWCNT. By comparing the samples of Nano DF/RPGF and Nano DF/RPG.GEF, it is found out that PEG in the presence of MWCNT can improve the shale recovery, because PEG is absorbed on shale surface and makes it stable.
3.3. Shale Integrity Test Results
In Fig. (24), the shale samples after the shale integrity test is shown. In this test, Nano DF/RMG.GEF is used as a WBM. According to Fig. (24), it can be found that the shale tablet is crushed, yet there is no scatteration. MWCNT prevents shale sample from dispersion and scatteration. Clearly, WBM cannot maintain the shale sample akin to OBM. Shale destruction could be reduced by raising the concentration of PEG in WBM.
 |
Fig. (24). Shale Samples after shale integrity test. |
According to Article SPE28818 and the previous literature, in order to control the shale integrity, the concentration of PEG must be 3% (weight/volume), e.g. 10.5 gr / 350 ml [23], while, in the present study, PEG is used with the concentration of 0.2% (0.75 gr / 350 ml) to reveal the effects of MWCNT on the shale stability.
CONCLUSION
According to the tests in the following, the experimental results have been described. The performance of MWCNT in the presence of salt (local water) is less than distilled water. In the presence of MWCNT, addition of bentonite before salt increases the viscosity of WBM. In the presence of MWCNT, by using GA as the surfactant decreases the water loss and increases the cake thickness of WBM. By adding MWCNT and PEG alone or together have different effects on the rheological properties of WBM. The addition of MWCNT alone almost improves the rheological properties of WBM, while the addition of PEG alone reduces the rheological properties of the base mud. Furthermore, the addition of PEG to the sample consisting of MWCNT reduces the viscosity and the yield point of WBM. In addition, the viscosity and the yield point of those samples containing MWCNT and PEG are higher than those samples consisting of PEG alone. By increasing the size (diameter and length) of MWCNT, the viscosity, pH and the yield point of WBM increase. MWCNT improves the shale integrity. PEG improves the shale recovery in the presence of MWCNT. Hence, MWCNT prevents the shale sample from any disintegration.
NOTES
1 Department of Petroleum Engineering, Faculty of Engineering, Islamic Azad University-Central Tehran Branch, Tehran, Iran; *Corresponding author Email: koorosh2kalloo@yahoo.com
2 Research Institute of Petroleum Industry (RIPI), Tehran, Iran, heidarianj@yahoo.com
3 Research Institute of Petroleum Industry (RIPI), Tehran, Iran, soleymanim@ripi.ir
4 Research Institute of Petroleum Industry (RIPI), Tehran, Iran, rashidiam@ripi.ir
5 Department of Petroleum Engineering, Faculty of Engineering, Islamic Azad University-Central Tehran Branch, Tehran, Iran, mahdinazarisaram@yahoo.com
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The author would like to acknowledge Mr. Mohammad Tavana and Mr. Kazemzadeh and the support of Research Institute of Petroleum Industry of Iran (RIPI) for conducting the present research.




