RESEARCH ARTICLE
Evaluating the Effects of Monovalent and Divalent Salts on the Rheological Properties of Water Based Mud
Ohenewaa K. Dankwa1, *, Prince Opoku Appau2, Eric Broni-Bediako1
Article Information
Identifiers and Pagination:
Year: 2018Volume: 11
First Page: 98
Last Page: 106
Publisher Id: TOPEJ-11-98
DOI: 10.2174/1874834101811010098
Article History:
Received Date: 15/1/2018Revision Received Date: 15/5/2018
Acceptance Date: 11/9/2018
Electronic publication date: 18/10/2018
Collection year: 2018
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction:
Drilling fluid selection plays a key role in preventing major problems encountered during drilling operations such as hole pack-off, stuck pipe and loss circulation. Mud contamination which results from the overtreatment of the mud system with additives or foreign material entering the mud system during drilling operations causes unwanted changes in the properties of the mud. This makes the mud system inefficient in performing its major roles. This research studies the effects of monovalent and divalent salts namely Potassium Chloride, Calcium Chloride, and Magnesium Chloride on the rheological properties of water-based mud system which is most vulnerable to contamination.
Methods:
Sixteen mud samples were formulated of which fifteen were contaminated each with different concentrations (0.75 g, 1.50 g, 2.50 g, 3.50 g, and 5.0 g) of the various salts at ambient temperature.
Results:
The results showed that the rheological properties such as plastic viscosity, apparent viscosity and yield point of the mud samples decreased as the concentrations of various salts increase.
Conclusion:
It was concluded that increase in the concentration of the salts resulted in a decrease in the rheological properties of the mud samples. This indicates that with the monovalent and divalent salt contamination, there is a significant decline in the performance of drilling mud since the salts affect the dispersion, hydration and flocculation behaviour of the particles. The effect was more profound with CaCl2 and MgCl2 salts than the KCl salt.
1. INTRODUCTION
Most wells are drilled with clear water for faster penetration rates, until a depth is reached where hole conditions dictate a need for a fluid (drilling fluid) with special properties. Drilling fluid or basically, mud is usually a mixture of water, clay, weighing material and some few chemicals [1]. Drilling fluids are usually formulated to meet certain properties to enable the mud to carry out its basic functions. A properly designed drilling fluid should be able to perform some major functions such as controlling formation pressures, removing cuttings from the wellbore, sealing permeable formations encountered while drilling, cooling and lubricating the bit, transmitting hydraulic energy to downhole tools and the bit and perhaps most importantly maintaining wellbore stability so as to meet up with the design objectives [2]. According to the World Oil annual classification of fluid systems, there are nine distinctive categories of drilling fluids that are in use today [3]. However, drilling fluids are usually classified based on the continuous phase (water-based fluids, oil-based fluids and pneumatic (gas) fluids) with the continuous phase determining the type of drilling fluid [4]. Each type of drilling fluid is designed specifically to fit a particular formation characteristics, therefore not the same type of fluid is used to drill all formations. For the purpose of this research, water-based mud is considered because it is cheap in relation to cost and formulation as well as environmentally friendly as compared to the other drilling fluids. Water-based mud can further be divided into three sub classifications namely, inhibitive; non-inhibitive and polymer water-based mud [5]. Therefore a non-inhibitive water-based drilling fluid (water-based mud without the presence of sodium, calcium and potassium ions) was designed to achieve the mud’s primary functions as mentioned earlier. Although it is the mud engineer’s motive to prepare a fluid that is capable of performing its functions during the drilling operation, but unfortunately that is not the case for actual field operations.
During drilling operation, the drilling mud picks up contaminants such as salts, drill solids, and cement. The instability in the drilling mud properties is as a result of various contaminants incorporated in the mud system [6]. Thus, a high concentration of contaminants in a drilling mud system causes a detrimental effect on its performance [7]. In general, mud is contaminated when any material that causes undesirable changes in the properties of the drilling mud enters the mud system. Solids, cement, sodium chloride, calcium or magnesium, salts, carbonates and bicarbonates, and anhydrite-gypsum are contaminants that are usually encountered during drilling operations either from the drilling or water. Sodium Chloride (NaCl) is the most common salt encountered during drilling operations. When this salt gets in contact with the drilling mud, it will flocculate the mud system, lower the pH, and likewise affect the properties of the drilling fluid. All of these contaminants cause a detrimental effect on the mud system thereby limiting its ability to perform its basic functions and also resulting in slow drilling rate [8]. According to Bourgoyne et al. (1986), drilling fluid is linked directly or indirectly to most of the drilling problems [9]. Solids are by far the most predominant contaminants. Excessive solids, whether commercial or obtained from the formation lead to high rheological properties and slow the drilling rate. The extent of the effects of contamination depends largely on the mud type, concentration and the type of the contaminant. Moreover, the contact of salt with drilling mud affects the degree of drilling fluid efficiency and its performance in drilling operation is affected by its rheological properties so it is necessary to study the drilling mud rheological parameters and properties at the downhole condition [10].
Rheology is the study of the deformation of fluids and flow of matter. Rheological properties also describe the flow characteristics of a mud under different flow conditions. In order to know the effects of flow, it is important that the flow behaviour of the mud at various points of interest in the mud circulating system is known [11]. Drilling muds should be designed with several suitable characteristics to improve the efficiency of the drilling operation. The most common characteristics are the rheological properties (like plastic viscosity, yield value, gel strengths and filter cake), stability under various operating conditions and stability against contaminating fluids [12]. Plastic viscosity is that part of the resistance to flow caused by mechanical friction. The friction is caused by solids concentration, size and shape of solids and viscosity of the fluid phase. The Yield Point (YP) is the initial resistance to flow caused by electrochemical forces between the particles. This electrochemical force is due to charges on the surface of the particles dispersed in the fluid phase. The YP indicates the ability of the drilling mud to carry cuttings to surface. Changes in PV of the mud can cause changes in YP, so the yield point should not be kept too low so as to suspend the cuttings at static conditions and also when it is pumped out of the annulus [13]. On the other hand, high-temperature environment tends to increase the YP of the water base mud too high because of the presence of contaminants such as carbon dioxide, salt, and anhydrite in the drilling fluids thereby limiting the mud efficiency [14]. Apparent viscosity is the viscosity of a fluid measured at a given shear rate and at a fixed temperature. It is a reflection of the plastic viscosity and yield point combined. An increase in the yield point and plastic viscosity will cause a rise in apparent viscosity. This is sometimes called single point viscosity [15]. Therefore when the salts contact the drilling fluid, the double layer of the clay particles is compressed enhancing flocculation of the suspension. In other words, the separation between the clay platelets was reduced with increasing concentration of salt. It will decrease the viscosity of the drilling fluid [16]. Divalent salts (calcium and magnesium) will have a greater contaminating effect on water-based muds than monovalent salts (sodium and potassium) [6].
The rheological parameters help to characterize the behaviour of the mud and its capacity to execute the desired functions, therefore salt contamination has been a burden to mud engineers as to how to deal with the effects these contaminants have on the properties of the drilling fluid. This situation needs a research to be conducted in an effort to identify the effects of these contaminants on the mud and how it can be treated. Luckily, over the years, numerous scholars have investigated in this phenomena to help provide comprehensive knowledge on salt contamination and how they impact the rheological properties of water-based mud. Ali et al. (2013) investigated the effect of NaCl salt contamination on the rheological properties of bentonite drilling mud and concluded that both plastic viscosity and the electrical resistivity were reduced with an increase in salt content [17]. According to Joel (2013), rheological properties of mud samples contaminated with three different salts namely NaCl, CaCl2 and KCl showed that the rheological properties were reduced with increase in salt concentration [18]. Hassiba and Amani (2013), investigated the effect of different electrolysis (NaCl, KCl) on the viscosity of water-based mud at high-pressure high-temperature conditions. Their research revealed that NaCl contamination increases the shear stress/shear rate curves of water-based mud; whereas KCl contamination decreases the shear stress/shear rate curves of water-based mud [19]. In other words, the amount of force required to carry the cuttings from the bottom of the well, through the annulus of the well and back to the surface will be reduced because of these foreign materials (salts) incorporated in the drilling mud thereby limiting the mud function as a cutting transporter. Another published work by other researchers on the effects of salt contamination on water-based drilling fluid performance demonstrated a decrease in the rheological properties of the mud samples as the salt concentration increased [20-23].
Although these researchers investigated the effects of salts contamination on the rheological properties of water-based system but their approach had some limitations. The water-based systems used in their experiment had a reactive phase which has the capacity to influence the rheology of the mud. Also the salt concentration they assumed was of high value, thereby neglecting the least effect the mud would have upon contact with the salt. Therefore, there is a need to investigate the least concentration of salt using a nonreactive phase water-based fluid. This study then focuses on the effect of potassium, calcium and magnesium salts contaminants at different concentrations on the efficiency of water-based drilling mud rheological properties (plastic viscosity, apparent viscosity and yield point). However, NaCl was not considered because a lot of researches have been reported in the literature about its effects on mud properties. Therefore, there is a need to investigate other types of monovalent and divalent salts on the properties of water-based mud. Although, literature suggests that temperature plays a crucial role when it comes to salt contamination since it has the capability to negatively affect the rheological properties at downhole conditions but not all oil wells are high-temperature highly pressured. Specifically in this work, temperature effect was not considered because the focus of this research is to study the effect of the aforementioned salts on the rheological properties of the drilling mud samples at ambient conditions. The reason for conducting this research under ambient conditions was to provide the mud engineer with an idea of how the mud prepared will behave before it is used in the field, and with this information they can have a fair idea of the nature of the fluid system even before it is pumped downhole so that modifications can be made in the prepared mud. This will in turn prevent drilling problems that result from the inefficiency of drilling mud to perform it major roles ahead of time to save time and cost.
2. MATERIALS AND METHODS
2.1. Materials Used
In this research work, three different types of salts were used in the formulation of the water-based drilling mud to determine their effects on the rheological properties of the mud system. The three types of salts are potassium chloride, calcium chloride and magnesium chloride which were received from Halliburton Ghana. Their respective proportion of purity was 99% for potassium chloride salt, 94-97% for calcium chloride and 98% for the magnesium chloride salt. Also the commercial bentonite used in the mud preparation for serving as a viscofier was also received from Halliburton Ghana, and its physicochemical properties also conformed to the API standard bentonite for drilling as shown in Table 1-1. Tap water was used as the mixed water in the mud preparation. Table 1 shows the quantity of materials used in the mud samples preparation.
| Materials | Quantity |
|---|---|
| Commercial Bentonite | 360 g (22.5 per set-up) |
| Mix Water (Fresh water) | 5 600 ml (350 ml per set-up) |
| Potassium Chloride (99% purity) | 13.25 g (0.2, 0.4, 0.7, 1.0 and 1.4% conc.) |
| Calcium Chloride, (94-997% purity) | 13.25 g (0.2, 0.4, 0.7, 1.0 and 1.4% conc.) |
| Magnesium Chloride, (98% purity) | 13.25 g (0.2, 0.4, 0.7, 1.0 and 1.4% conc.) |
| Product | Form | Absolute volume L/kg (gal/lb) | Molecular weight (g/mol) | Specific gravity |
Flash Point (degC) [degF] |
pH |
|---|---|---|---|---|---|---|
| Sodium Bentonite | Light Tan to Gray Powder | 0.376 (0.045) |
421.8 | 2.85 | > 93 [> 200] |
< 9.5 |
| Mineral Composition (%) | ||||||
| Na2O | Al2O3 | Fe2O3 | MgO | CaO | SiO2 | K2O |
| 2.02 | 17.01 | 6.51 | 1.92 | 1.37 | 66.12 | 0.56 |
2.2. Experimental Procedure
Petri dish was used to weigh a total of 360 g of the Bentonite on an electronic balance. Measuring cylinder was used to measure the total quantity of 5,600 ml of fresh water. 22.5 g of bentonite and 350 ml of fresh water were used in each of the set-up according to API requirement for preparing drilling fluid. Initially, half (2,800 ml) of the fresh water was poured into a mixing cup and the weighed bentonite was added. The handheld mixer was used to stir the mixture in the container for a while. The remaining water was added and stirred with the handheld mixer for 5 minutes so as to ensure homogeneity. The mixture was allowed to stand for 16 hours minimum for the bentonite to swell. Sixteen experimental set-ups were necessary for this investigation.
After the bentonite was swollen, different concentrations (0.2%, 0.4%, 2.5%, 3.5%, and 1.4%) of the individual monovalent and divalent salts (KCl, CaCl2 and MgCl2) were weighed and added to the bentonite. Sixteen samples were formulated which include one control sample, of which, no salt was added. The remaining fifteen samples were contaminated with the various salts, five samples for each salt. The samples were left for about two hours for the salt to react well with the mud. Throughout the laboratory experiment, each mud sample assumed a volume of 350 ml. The dial readings of the various samples were taken by the use of the Fann Viscometer Model 3500 at readings of 600, 300, 200, 100, 60, 30 and 3 rpm as indicated by API to measure the rheological properties of mud samples. Overall, to ensure the accuracy of the experimental results, API standard procedures for preparing mud and determining the mud properties were followed throughout the experimental work. The plastic viscosity, apparent viscosity and yield point values were calculated using Equation 1, 2 and 3 respectively [4]:
Plastic Viscosity (µp) in mPa.s
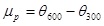 |
(1) |
Yield Point (YP) in (lb/100ft2)
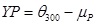 |
(2) |
Apparent Viscosity (µa) in mPa.s
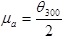 |
(3) |
where; is the dial reading at 600 rpm and is the dial reading at 300 rpm
3. RESULTS AND DISCUSSION
To ensure the validity of the experiment performed, the analysis of the results was conducted based on comparative analysis using a control mud sample and America Petroleum Institute (API) specification standard for drilling fluid as well as already conducted research work validated results. Table 2 shows the API 13A specification requirement for rheological parameters for the use of drilling grade bentonite in mud preparation.
| RHEOLOGICAL PROPERTIES | |
|---|---|
| Ɵ600 RPM | 30 Minimum |
| Ɵ300 RPM | 23 Minimum |
| PLASTIC VISCOSITY | 6-8 Minimum |
| APPARENT VISCOSITY | 12-15 Minimum |
| YIELD POINT | 3*PV or 50 Maximum |
| YIELD POINT/PLASTIC VISCOSITY | 3 Maximum |
Table 3 presents a total account of results from the rheological tests conducted at the laboratory on the mud samples giving details of the rpm readings. Table 3 also shows the calculated values of plastic viscosity, yield point and apparent viscosity. The experiment was also conducted at room temperature which was considered to be 25 °C, but in the course of the experiment, it is not certain that the temperature remains constant at 25 °C, thus, this uncertainty can also have an influence on the results obtained. The bentonite and salts samples were weighed with an electronic balance which has the capability to determine the exact amount of each material used in the mud formulation to the nearest mark. Hence it was assumed that the uncertainty of the measured sample is zero since the external factor such as wind blowing was not considered to have an influence on the readings. However, during the computations of each amount of salt used in terms of concentration, some of the values were corrected to the nearest two decimal place The rheometer was placed on a flat desk in order to set the rheometer mark at zero before taking the readings in order to avoid error readings. However, human errors can be introduced into the results through the reading from the equipment. Also, the rheometer used in taking the dial readings was assumed to be accurate since several readings at particular dial reading gave the same value. The uncertainties of the experimental data in Table 3 is in the range of ± 0.01.
| PARAMETERS | WATER BASED DRILLING FLUID SAMPLES (25 °C) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MUD | MUD + KCl salt | MUD + CaCl2 salt | MUD + MgCl2 salt | |||||||||||||
| % Concentration of Salt | ||||||||||||||||
| 0.0 | 0.2 | 0.4 | 0.7 | 1.0 | 1.4 | 0.2 | 0.4 | 0.7 | 1.0 | 1.4 | 0.2 | 0.4 | 0.7 | 1.0 | 1.4 | |
| Ɵ600 RPM | 24 | 28 | 26 | 24 | 23 | 21 | 14 | 10 | 9 | 9 | 8 | 14 | 10 | 10 | 9 | 9 |
| Ɵ300 RPM | 17 | 20 | 19 | 17 | 16 | 15 | 10 | 7 | 6 | 6 | 5 | 9 | 7 | 7 | 6 | 6 |
| Ɵ6 RPM | 11 | 12 | 13 | 11 | 10 | 9 | 5 | 3 | 2 | 2 | 2 | 5 | 2 | 1 | 1 | 1 |
| Ɵ3 RPM | 10 | 11 | 9 | 9 | 8 | 8 | 4 | 3 | 2 | 2 | 1 | 4 | 2 | 1 | 1 | 1 |
| PV (mPa.s) | 7 | 8 | 7 | 7 | 7 | 6 | 4 | 3 | 3 | 3 | 3 | 5 | 3 | 3 | 3 | 3 |
| AV (mPa.s) | 12 | 14 | 13 | 12 | 11.5 | 10.5 | 7 | 5 | 4.5 | 4.5 | 4 | 7 | 5 | 5 | 4.5 | 4.5 |
| YP (lb/100ft2) | 10 | 12 | 12 | 10 | 9 | 9 | 6 | 4 | 3 | 3 | 2 | 4 | 4 | 4 | 3 | 3 |
3.1. Effect of KCl, CaCl2 and MgCl2 Concentration on Plastic Viscosity
Fig. (1) shows the effect of plastic viscosity against each salt at different salt concentrations. In Fig. (1), there is a decrease in plastic viscosity as the different salt concentrations increase. API specifications for drilling fluid as depicted in Table 2 suggests that the minimum plastic viscosity needed for efficient drilling should be of a value of 7 mPa.s. The control mud met the API standard, but this situation was not seen in the mud contaminated with various salts since the plastic viscosity of mud with Potassium Chloride (KCl) salt decreased from the range of 8 to 6 mPa.s and that of mud with Calcium Chloride (CaCl2) and Magnesium Chloride (MgCl2) salt, both reduced from 7 to 3 mPa.s for salt concentrations of 0.2%, 0.4%, 0.7%, 1.0% and 1.4%. Although reduced PV indicates that the mud is capable of drilling rapidly because of low viscosity of the mud exiting the bit, nevertheless, the plastic viscosity must be maintained in the range of 6 - 8 mPa.s for a successful drilling operation. This is because low PV implies low Equivalent Circulating Density (ECD) exerted at the bottom while high PV triggers an increase in ECD because high pumping is required to break the gel [25].
 |
Fig. (1). A plot of Plastic Viscosity against Different Salt Concentrations. |
3.2. Effect of KCl, CaCl2 and MgCl2 Concentration on Yield Point
Similarly, Fig. (2) shows the effect of the yield point (YP) against each salt at different salt concentrations; and it indicates a decrease in yield points of the mud samples as the different salt concentrations increase. API standard recommends that the yield point of a drilling mud should have a numerical value three times the plastic viscosity or not exceed to the value of 50 for optimal drilling. Unlike the control mud which had a yield point value of 10 lb/100ft2 which almost meets the API standard, the yield point of the mud with KCl salt decreased from 12 lb/100ft2 to 9 lb/100ft2. Also yield points of mud with CaCl2 and MgCl2 salts decreased in the range of 10 lb/100ft2 to 2 lb/100ft2 and 10 lb/100ft2 to 3 lb/100ft2 respectively. For mud with CaCl2 and MgCl2, yield points decreased as salt concentrations increased unlike the mud with KCl salt where the yield point stabilised and decreases as salt concentration increased. This occurrence is due to the inhibitive nature of potassium ions because of its lower cation replacing power. Yield point shows the ability of drilling mud to carry cuttings to surface, therefore a reduction in its numerical value renders the mud ineffective in carrying out its role as a cutting transporter. In addition, it is very important to keep the value of YP/PV ratio of the drilling fluid high enough (maximum of 3) to ensure effective cutting transport.
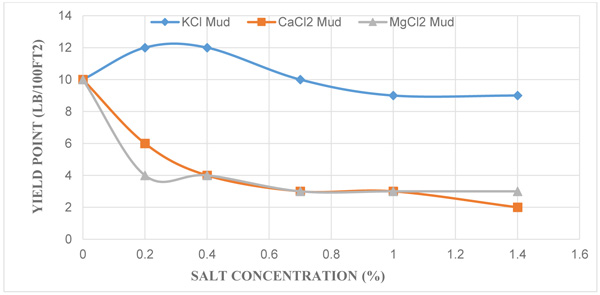 |
Fig. (2). A plot of Yield Point against Different Salt Concentrations. |
3.3. Effect of KCl, CaCl2 and MgCl2 Concentration on Apparent Viscosity
Fig. (3) depicts the effect of apparent viscosity (µa) against each salt concentrations where it gives an indication that an increase in the various salt concentrations resulted in the decrease in apparent viscosities of the contaminated mud samples. The control mud with 12 mPa.s which was also used as a standard conformed to the API requirement for using drilling grade bentonite mud which dictates that the apparent viscosity should have a minimum value in the range of 12 to 15 mPa.s. On the other hand, the apparent viscosities recorded reduced from the range of 14 mPa.s to 10.5 mPa.s for mud samples with KCl salt, 12 mPa.s to 4 mPa.s for mud samples with CaCl2 salt and then 12 mPa.s to 4.5 mPa.s for mud samples with MgCl2 salt at concentrations of 0.2%, 0.4%, 0.7%, 1.0%, and 1.4%. Apparent viscosity is the viscosity at 600 rpm reading, and is the reflection of both the plastic viscosity and yield point. This shows that a decrease in both of them will result in a decrease in the apparent viscosity. This value helps the mud engineer develop and maintain the properties of the drilling fluid to the specifications required.
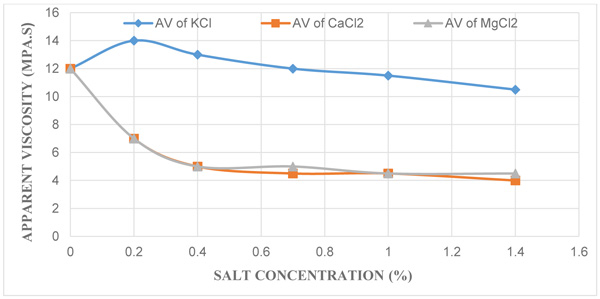 |
Fig. (3). A plot of Apparent Viscosity against Different Salt Concentrations. |
Potassium ions compared to other ions are unique in nature. Due to their inhibitive property, little or no effect can be shown when added to the drilling fluid. This is because potassium ions tend to fit much closer into the lattices of clay structure, thereby reducing its ability to hydrate [6]. The ion exchange model does not fully explain the interaction of potassium with clay [18]. Calcium and magnesium have a greater cation exchange replacing power than sodium. When bentonite is added to water containing more than one hundred ppm of either of these ions, the degree of hydration is greatly reduced and the tendency to disperse is inhibited. As a result, very little viscosity is developed and the fluid loss is quite high [1].
Already conducted and validated studies by various researchers on the effect of salt on the properties of water-based mud is in agreement with the findings of this research in the sense that their work depicted the same trend with this research work as shown in Fig. (1) through to Fig. (3). In their work, it was observed that the increase in salt concentrations resulted in a decrease in the rheological properties of the mud samples [20, 18, 23]. Also in this present study, the results indicated that with the smallest concentration of the salt as 0.2%, there was a decrease in the rheological properties, however, the effect was significant with the mud contaminated with divalent salts (CaCl2 and MgCl2) than the mud contaminated with the monovalent salt (KCl)
The general results indicated that cation might aid as a bond to hold the clay mineral particles together. According to Weber (1970), multivalent cations have the potential to bond layers together more firmly than monovalent cations, usually resulting in aggregation of the clay particles. However, potassium, a monovalent cation, is the exclusion to the rule [26]. The ionic content of water affects the hydration, dispersion and flocculation behaviour of clay particles [27]. As the salt content is increased, the degree of hydration and dispersion is decreased. This is caused by the cations in solution pushing the exchange cations closer to the surface of the clay platelet. This causes the water layer bound by the clay surface and the exchange cations to be thinner. As a result, the viscosity will be less. Since the water layer is thinner, the platelets can come closer to one another and their tendency to flocculate will be greater [28].
CONCLUSION AND RECOMMENDATIONS
The following conclusions can be drawn from this study:
- This study has shown that the rheological properties of the water-based mud were affected by the various salts contamination which will, in turn, affect the efficiencies of the mud samples.
- Overall, the increase in the concentrations of the various salts decreased the plastic viscosities, apparent viscosities and yield points of the contaminated mud samples.
- The effects of the salts on the mud samples were more profound on the mud samples contaminated with calcium and magnesium salts than the mud samples with potassium chloride salt.
At the end of this study, the contaminants affected the rheological properties of the mud samples, hence drilling mud free of contaminants is significant in any drilling program in order for the mud to execute its basic functions. Therefore, it is imperative for the mud engineer to have detailed knowledge about the formation to be drilled and the likely contaminants to be encountered forehand before the desired drilling fluid can be designed to combat the challenges to avoid drilling problems which will, in turn, save time and cost.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors appreciate the University of Mines and Technology (UMaT), Tarkwa for the supports granted them to complete this work.
REFERENCES
| [1] | Annis MR, Smith MV. Drilling fluids technology Revised Edition. 1974. |
| [2] | Yunita P, Irawan S, Kania D. Optimization of water-based drilling fluid using non-ionic and anionic surfactant additives. Procedia Eng 2016; 148: 1184-90. [https://doi.org/10.1016/j.proeng.2016.06.628]. |
| [3] | World Oil. Drilling, Completion and Workover Fluids Gulf Publishing Company 2015.https://www.worldoil.com/media/ 2464/fluids_tables.pdf |
| [4] | Darley HCH, Gray GR. Composition and properties of dilling and completion fluids 7th ed. 2017. [https://books.google.com/books?id=kS6lCgAAQBAJ] |
| [5] | Reza M. Effect of Nano silica in brine treated PAC/ XC/LV-CMC polymer –Bentonite fluid system MS Thesis, University of Stavanger, 2015. |
| [6] | Joel OF, Durueke UJ, Nwokoye CU. Effect of cement contamination on some properties of drilling mud Nigeria Annual International Conference and Exhibition Abuja, Nigeria. 2012; pp. 2012; 1-5. |
| [7] | Broughton G, Hand RS. Viscosity characteristics of clays in connection with drilling muds. J Pet Technol 1938; 1(4): 1-7. [https://doi.org/10.2118/938112-G]. |
| [8] | Joel OF. Design and field application of drilling, cementing and stimulation fluids 2010. |
| [9] | Bourgoyne AT, Millheim KK, Chenevert ME, Young FS. Applied Drilling Engineering 1986; Vol. 2 |
| [10] | Nasser J, Jesil A, Mohiuddin T, Al-Ruqeshi M, Devi G, Mohataram S. Experimental Investigation of Drilling Fluid Performance as Nanoparticles. World Journal of Nano Science and Engineering 2013; 3(3): 57-61. |
| [11] | Awele N. Investigation of Additives on Drilling Mud Performance with, Tonder Geothermal, Drilling as a Case Study MS Thesis 2014. |
| [12] | Amani M, Al-Jubouri M, Shadravan A. Advanced petroleum exploration development 4th ed. 2012; 2 |
| [13] | Ogbeide PO, Igbinere SA. The effect of additives on rheological properties of drilling fluid in highly deviated wells. Futo Journal Series 2016; 2(2): 68-82. [FUTOJNLS]. |
| [14] | Azar JJ, Samuel GR. Drilling engineering 2007. |
| [15] | Annudeep SD. Rheological Properties and Corrosion Characteristics of Drilling Mud Additives MS Thesis 2012. |
| [16] | Alaskari MKG, Teymoori R. Effects of Salinity, pH and Temperature on CMC Polymer and XC Polymer Performance International Journal of Engineering Transactions B: Applications 2007; 20(3): 283-90. |
| [17] | Ali K, Vipulanandan C, Richardson D. Salt (NaCl) contamination on the resistivity and plastic viscosity of a bentonite drilling mud Proceedings of CIGMAT Conference & Exhibition 2013; 1-2. |
| [18] | Joel OF. Comparative study of different salts on hydration of bentonite Nigeria Annual International Conference and Exhibition Lagos, Nigeria. 30th July - 1st August, 2013; 1-10. |
| [19] | Hassiba KJ, Amani M. The Effect of salinity on the rheological properties of water based mud under High temperatures for drilling offshores and deep wells. Earth Sci Res J 2013; 2(1): 175-86. [http://dx.doi.org/10.5539/esr.v2n1p175]. |
| [20] | Joel OF, Durueke UJ, Nwokoye CU. Effect of KCl on rheological properties of shale contaminated Water-Based Mud (WBM). Global Journal of Researches in Engineering 2012; 12(9): 1-9. |
| [21] | Adekomaya OA. Experimental analysis of the effects of magnesium saltwater influx on the behaviour of drilling fluids. Journal of Petroleum Exploration And Production Technology 2013; 3(1): 61-7. |
| [22] | Ichenwo J, Okotie S. Experimental investigation on the effect of KCL and bentonite on shale stability. International Journal of Current Engineering and Technology 2015; 5(1): 959-65. |
| [23] | Sami NA. Effects of magnesium saltwater contamination on the behaviour of drilling fluids. Egyptian Petroleum Journal 2015; 1(1): 1-6. [https://doi.org/10.1016/j.ejpe.2015.10.011]. |
| [24] | API SPEC 13A, Specification for Drilling Fluids – Specifications and Testing, 18th ed, Purchasing Guidelines Handbook, American Petroleum Institute (API), 2010. |
| [25] | Irawan S, Kinif BI, Bayuaji R. Maximizing Drilling Performance through Enhanced Solid Control System IOP Conf Series: Materials Science and Engineering 2017; 267(012038): 1-15. |
| [26] | Weber JB. Adsorption of s-triazines by montmorillonite as a function of pH and molecular structure. Soil Science Society of America Proceedings 1970; 34: 401-4. |
| [27] | Rogers WF. Composition and properties of oil well drilling fluids 3rd ed. 1963. |
| [28] | Goud M. Mud Engineering Simplified, BecomeShakespeare.com, ISBN: 9789386487674, 2017. [https://books.google.com/ books?id=9P5CDwAAQBAJ]. |




