RESEARCH ARTICLE
Study of Influences of Fracture Additives on Stability of Crude Oil Emulsion
Hongbo Fang1, Mingxia Wang2, *, Xiaoyun Liu1, Weinan Jin3, Xiangyang Ma3, Xiangyu Meng3, Feng Yan3, *
Article Information
Identifiers and Pagination:
Year: 2018Volume: 11
First Page: 118
Last Page: 128
Publisher Id: TOPEJ-11-118
DOI: 10.2174/1874834101811010118
Article History:
Received Date: 25/7/2018Revision Received Date: 2/11/2018
Acceptance Date: 2/11/2018
Electronic publication date: 30/11/2018
Collection year: 2018
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
A hydraulic fracture is a key technology to increase production of the low permeability oil fields. Fracture additives such as gels, friction reducers, pH adjusters and clay stabilizers were injected into the underground. While more than 50% of the fracture fluid remains underground. The residue of fracture fluid comes out with the produced liquid (a mixture of crude oil and water) in the subsequent oil recovery process, which results in a highly stable crude oil-water emulsion.
Objective:
The stability and stable mechanism of the emulsion with fracture fluid have been experimentally investigated.
Materials and Methods:
The influences of fracture additives and components of crude oil on the stability of emulsion were investigated by bottle test and microscopic examination. The interfacial tension and modulus of dilation were explored by a spinning drop interfacial tension meter and an interface expansion rheometer, respectively.
Results:
The fracture additives played the key role on the emulsion stability. On one hand, the interface energy of oil-water was reduced by friction reducer (IFT was decreased from 24.0 mN/m to 1.9 mN/m), which was a favor for the formation of an emulsion. On the other hand, the dilational modulus of crude oil-water film was increased by hydroxypropyl guar and pH adjuster (Na2CO3) to form a viscoelastic film, which resulted in a highly stable emulsion.
Conclusion:
The residual fracture fluid accompanied by produced liquid resulted in a highly stable emulsion. The emulsion with fracture additives was difficult to be broken, which may affect the normal production of the oil field. A positive strategy such as developing demulsifier with high efficient should be put onto the schedule.
1. INTRODUCTION
Low-permeability oil reservoirs have become one of the most important sources of energy in China and also in North America [1-3]. Due to the low porosity and low permeability of this reservoir, hydraulic fracturing treatments combined with horizontal wells are widely applied in low-permeability reservoirs [4-6]. Hydraulic fracturing (HF) is a technique where fluids are pumped into wells under high pressure in order to increase formation permeability [7, 8]. HF is commonly applied to increase permeability in shale, tight sands, and other oil-bearing strata, resulting in higher oil production [7].
Typical hydraulic fracture additives include gels, friction reducers, crosslinkers, pH adjusters, clay stabilizers and breakers. Gels are used in fracturing fluids in order to increase the viscosity of the liquid, thus enhancing the proppant (usually sand) to be suspended in the mix and ultimately penetrating in the geologic formations to hold open the cracks created by the hydraulic fracturing. The most common gelling agents are guar gum and its derivatives, such as carboxymethyl guar (CMG), hydroxypropyl guar (HPG), and carboxymethyl hydroxypropyl guar (CMHPG). Guar molecules are composed of β-1,4-linked mannose units with α-1,6-linked galactose units attached [9-11], where the ratio of mannose to galactose units is typically 1.6:1 to 1.8:1 [10, 11], and the average molecular weights range from 50,000 to 8,000,000. Crosslinkers are added to fracturing fluids to chemically bind individual gel polymer molecules together to form larger molecules, resulting in higher viscosity, more elasticity, and better proppant transport compared with linear gels that are not crosslinked. Crosslinkers frequently used in HF include borate salts; aluminum, titanium, and zirconium compounds; monoethanolamine; and monoethylamine. The concentrations of crosslinkers in fracturing fluid are relatively low and range from 0.5 to 250 mg L-1 [12]. Friction reducers are added to allow fracturing fluids and sand, or other solid materials called proppants, to be pumped to the target zone at a higher rate and reduced pressure than if water alone was used. A commonly used friction reducer is 2-propenamide (polyacrylamide), (C3H5NO)n. Reported application rates in fracturing fluids are 30–1200 mg L−1 [12]. Acids and bases are added to fracturing fluids to adjust pH and improve the effectiveness of certain chemical additives. Typical pH adjusters include acetic acid, potassium hydroxide, sodium hydroxide, sodium carbonate, and potassium carbonate. The typical concentrations for pH adjusters range from 100 to 300 mg L−1. Clay stabilizers are used to prevent the swelling of clays. Clay swelling and migration can cause borehole instability and can reduce reservoir rock permeability by up to 90% and reducing well productivity [13]. Clay stabilizers work via ion exchange, replacing the cations (such as Na+) in the clay with other positive ions (such as Ca2+ or Mg2+) that have a lesser tendency to become hydrated and swell the clay. Clay stabilizers include choline chloride, tetramethyl ammonium chloride, potassium chloride and sodium chloride. The concentrations range from 500 to 2000 mg L−1. Following fracturing, an enzyme or inorganic breaker is introduced to reverse crosslinking, which reduces the viscosity of gelled fluids. The breaker and disrupts polymers, result in reduced molecular weight and fluid viscosity in order to meet the requirement of the flow-back. The broken fluid was called gel breaking liquid. At temperatures below 66 ◦C, enzyme breakers can be used to break guar gels [10, 14], but under higher temperature condition and at pH above 10.5, the effectiveness of most enzymes is reduced. Inorganic breakers, such as ammonium, potassium, and sodium salts of peroxydisulfate, are used at concentrations ranging from 1 to 400 mg L−1 [15].
The fracture fluids are recovered onto the ground after being broken using a breaker. However, it is typically reported that less than 5-50% of the injected fluids can be recovered as “flowback” [16, 17]. More than 50% of the fracture fluid remains underground. The residue fracture additives accompanied by the produced liquid (mixture of crude oil and water) in the subsequent oil recovery process. It was found in the oil site production that the produced oil-water mixture containing fracture fluid became a stable emulsion. The stability of the emulsion was supposed to the contribution of the fracture additives [18, 19]. However, there were no studies about the influences of fracture additives on crude oil-water emulsion as far as we know.
Therefore, the present work focuses on the stability and stable mechanism of crude oil-water emulsion with fracture additives. The influences of components of fracture fluid and of crude oil on the emulsion stability, particle size distribution, the interfacial tension, and dilational modulus emulsion were investigated systematically. The results of the present work would benefit the demulsification of mixture of crude oil-water containing fracture additives.
2. EXPERIMENTAL SECTION
2.1. Materials
Gel (hydroxypropyl guar powder, GRJ-11), friction reducers (SL-P), clay stabilizer (FP-2), pH adjuster (Na2CO3) and crude oil with the density of 0.85 g/cm3 (at 30°C) were provided by ShengLi Oilfield Engineering Consulting Co., Ltd., China. Kerosene, CP, was purchased from Sinopec Shanghai Petrochemical Co. Ltd., China, and was used after column chromatography. Components of crude oil, including saturates, aromatic, resins and asphaltenes, were obtained by the four-component separation method as illustrated in our previous work [20].
2.2. Methods
2.2.1. Emulsion Stability Test
Preparation of simulated emulsion: A simulated emulsion of crude oil-water with fracturing was prepared in the laboratory by mixing of 10 mL oil phase and 10 mL water phase. The oil phase was a solution of crude oil or crude oil compositions in kerosene, and the water phase was simulated water (as shown in Table 1 with or without fracture additives. The mixture was sheared by an IKA T18 basic shear apparatus (IKA Corp., Germany) at 15,500 rpm for 5 min and then it was poured into a graduated test tube with a stopper. After shaking vigorously 200 times by hand, a stable simulated emulsion was formed [19, 21].
| Ions | Cl- | CO32- | HCO3- | K+ | Na+ | NH4+ | Mg2+ | Ca2+ | Salinity |
| Content / mg.L-1 | 5780.02 | 36.25 | 1621.67 | 74.79 | 4091.17 | 99.01 | 59.54 | 251.75 | 11261.37 |
Bottle Testing [19, 22, 23]: The separation of water/oil from the emulsions was evaluated by bottle testing (SY/T 5281-2000) at room temperature and the percentage of water/oil separation was visually quantified. The dehydration rate (DH) and deoiling rate (DO) were defined as equation (1):
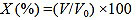 |
(1) |
Where X is the dehydration rate or deoiling rate which evaluates the stability of the emulsion. V is the volume of the water or oil separated; V0 is the original volume of water or oil contained in the emulsion.
2.2.2. Size Distribution of Droplets in Emulsion
The microscope is a particularly intuitive approach for calibration of droplet size distributions [24]. The size distribution of water droplets in the emulsions (W/O) was measured using a BK-POLR polarizing microscope (Chongqing Ott Optical Instrument Co., Ltd.) at room temperature. A freshly prepared emulsion drop was put on a glass slide, which was placed on the objective table of microscope rapidly. The images of the emulsion on the glass plate were taken. Then the particle size distribution of the droplets was determined.
2.2.3. Interfacial Tension
The interfacial tension is the basic parameter to evaluate the interfacial activity of the surface-active substance [25, 26]. The dynamic and equilibrium interfacial tensions (IFT) were determined by a high precision spinning drop interfacial tension meter (JJ2000B, Power each Ltd., Shanghai China). A little drop of oil phase was injected into a rotating capillary tube filled with the aqueous phase. The diameter of the elongated oil drop at a fixed temperature (T=30 °C) and speed of rotation (r=5000 rpm) was monitored as functions of time until it reached a constant value. From the drop diameter’s value, the dynamic and equilibrium IFTs were calculated by the software attached.
2.2.4. Dilational Interfacial Rheological Properties
The background of interfacial dilational viscoelasticity has been described in the literatures [27-29]. The interfacial viscoelasticity or interfacial dilational modulus (ε) is defined by
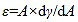 |
(2) |
where γ is the interfacial tension, A is the drop area and dA is the deformation.
In this study, the interfacial dilational viscoelasticity was measured by a JMP 2000A interface expansion rheometer (Power each Ltd., Shanghai, China). It includes a Langmuir trough with two symmetrically oscillating barriers for changing the interfacial area and a Wilhelmy plate for measuring the interfacial tension [21, 30]. The Langmuir trough was filled with 90 mL water phase and 60 mL oil phase (solution of crude oil in kerosene, 5 wt %). The dilational viscoelasticity experiment began after 6 h of pre-equilibrium. Then, the barriers were expanded and compressed sinusoidally with small amplitude in the frequency range of 0.0033 to 0.1 Hz. All experiments were performed at 30 °C.
3. RESULTS AND DISCUSSION
3.1. Emulsion Stability from a MacroscopicView
3.1.1. Effect of Oil Components on Stability of Emulsion
Different oil has different characteristic and offers different problems [31]. So the influences of oil components on emulsion stability were investigated. The oil components were extracted via column chromatography. The influences of oil components on the stability of emulsion were shown in Fig. (1). As shown in Fig. (1), the emulsions prepared from saturates-water and aromatics-water showed poor stability, because both emulsions achieved complete demulsification in a short time (less than 30 min). The emulsion prepared from resins-water was more stable than those from saturates-water and aromatics-water. 88.5% deoiling rate was observed, and no free water was obtained in more than 2 h. The emulsion prepared from asphaltenes was the most stable one. Less than 70% deoiling rate was achieved, and also no free water was obtained in near 3 h. Therefore, it can be concluded that the stability of emulsion is strongly correlated to asphaltenes and resins, namely, the resin and asphaltene make an important contribution to the emulsion stability among the oil components. These results were consistent with the previous work of Xia et al. [32]. They studied the influence of asphaltenes and resins on the stability and demulsification of emulsions and found that asphaltenes and the resins stabilize the emulsions, while in the absence of asphaltenes and resins, no stable emulsions can be formed in the water-jet kerosene system. The influence of asphaltenes and resins on emulsion stability from Ref [32]. were shown in Fig. (2). This is because that asphaltenes and resins adsorbed on the water-oil interface to form a viscoelastic interfacial film with high mechanical strength.
 |
Fig. (1). The emulsion stability of different oil components. Water phase: Simulated water; oil phase: crude oil components (a) deoiling rate, (b) dehydration rate. |
3.1.2. Influence of Fracture Additives on the Emulsion Stability
The influences of fracture additives on the stability of emulsion were studied in the present work. Fracture additives such as hydroxypropyl guar, friction reducers (SL-P), clay stabilizer (FP-2) and pH adjuster (Na2CO3) were dissolved in water. Then the water phase was mixed with oil phase (5% crude oil in kerosene) by an IKA T18 basic shear apparatus.
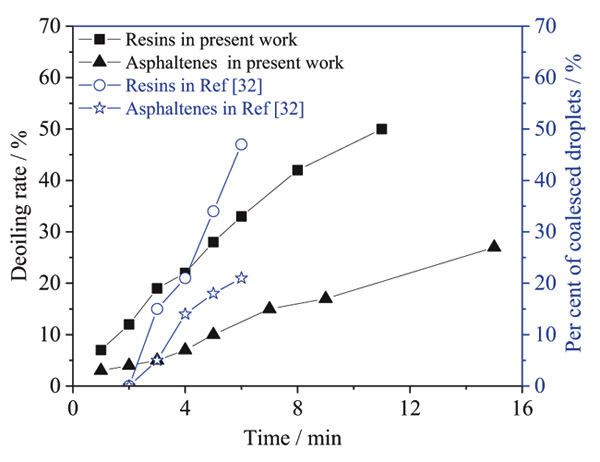 |
Fig. (2). The influence of asphaltenes and resins on emulsion stability (comparing with Ref [32].) |
The effects of fracture additives on emulsion stability were illustrated in Fig. (3). As shown in Fig. (3b), there was no free water observed for each emulsion, that is the dehydration rates were all 0% for emulsions prepared from water phases containing fracture additives and crude oil. However, there were some differences in deoiling rates. As shown in Fig. (3a), the deoiling rates at 120 min were 61%, 60%, 51% and 35% for emulsions with clay stabilizer, friction reducer, pH adjuster and hydroxypropyl guar, respectively. The results suggested that the emulsion with hydroxypropyl guar was the most stable one. Ferrer et al. [33] traced the chemical additives used in fracturing fluids by a high-resolution mass spectrometry coupled with liquid chromatography. Fragmentation of guar gum was detected in the flow back fluid. They supposed that guar gum may form a stable emulsion when dissolved in methanol or water, which was consistent with the present work. This may be due to enhancing the viscosity of the emulsion, and the amphiphilic properties of the hydroxypropyl guar. The improved viscosity of emulsion led to high frictional resistance and then prevented the collision between droplets of water droplets and slowing sinking speed to a large extent [17].
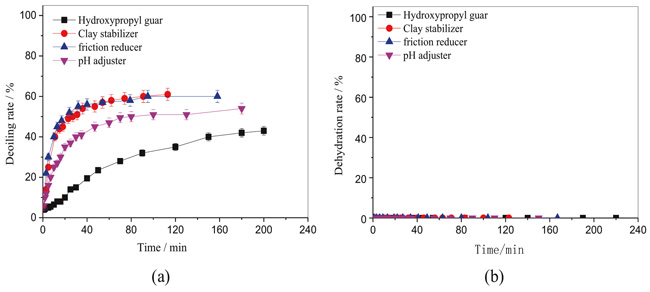 |
Fig. (3). The effect of fracture additives on the emulsion stability. Water phase: Simulated water with fracture additive; oil phase: 5% crude oil in kerosene (a) deoiling rate, (b) dehydration rate. |
3.1.3. Effect of the Interaction Between Oil Components and Gel Breaking Liquid
At the end of fracturing, the fracture fluid was broken by a breaker to reduce molecular weight and fluid viscosity. Therefore, the fluid that remains underground may mostly be the gel breaking liquid. Thus, it is important to investigate the influence of gel breaking liquid on the stability of emulsion. Herein, the influences of gel breaking liquid on the stability of emulsion prepared from oil components were explored as shown in Fig. (4). Similarly, the emulsions prepared from saturates-gel breaking liquid and aromatics-gel breaking liquid showed poor stability, because there were hardly any interfacial active substances in saturates and aromatics. The emulsions prepared from resins-gel breaking liquid and asphaltenes-gel breaking liquid were more stable. As shown in Fig. (4b), there were no free water phase separated for resins and asphaltenes in the experiment time range, and the deoiling rates for resins and asphaltenes were 79% and 58% in Fig. (4a), respectively. This result indicated that resin, asphaltene and gel breaking liquid, as well as their interaction, play important roles for the stability of emulsion. Zhang et al. [34] studied the demulsification of flow back fluid produced after fracturing in a low permeability oil field in Bohai China. They found that the flow back fluid was hard to be broken due to the serious emulsion, which was consistent with our observations, and can be illustrated according to the conclusion of this study.
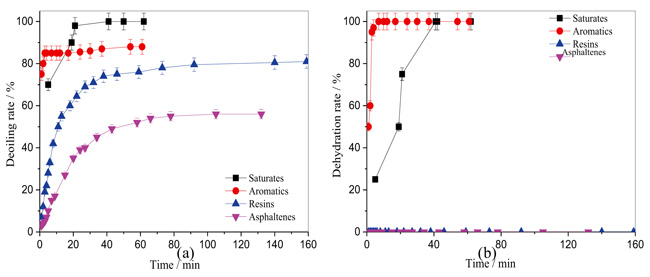 |
Fig. (4). The interactions between gel breaking liquid and ingredient of crude oil on emulsion stability. (a) deoiling rate, (b) dehydration rate |
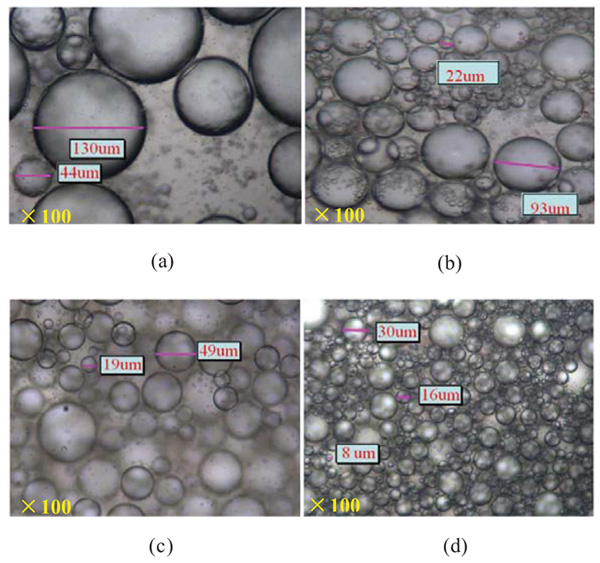 |
Fig. (5). The particle size distribution for various emulsion obtained by crude components. (a) saturates, (b) aromatics, (c) resins and (d) asphaltenes |
3.2. Inspection of Emulsion Particles
3.2.1. Effect of Oil Components
The initial droplet size is an indicator of emulsion stability. Generally, the smaller the initial droplet size is, the more stable the emulsion is. In this study, the effect of crude oil compositions on the stability of the emulsion was studied from the perspective of visual particle size under a polarizing microscope.
The influences of components of crude oil on the particle size of emulsion were shown in Fig. (5). As shown, the saturate emulsifier system had large particle sizes which mainly in 44μm~130 μm and the corresponding emulsion is unstable. The droplets sizes were mainly in 22 μm~93 μm for emulsion prepared from aromatics. The large distributes of the droplets size indicated unstable emulsions of the saturate and the aromatic. Furthermore, a smaller-sized droplet was obtained in resin emulsion, and the particle sizes mainly ranged from 19 μm to 49 μm. The smallest-sized droplet was obtained in asphaltenes emulsion as shown in Fig. (5d). There were well-distributed particles, and the particle sizes were mainly distributed in 9 μm~27 μm, which suggested that the asphaltene emulsion was very stable. These results agreed with the results reported in emulsion stability from a macroscopic view (Section 3.1.3).
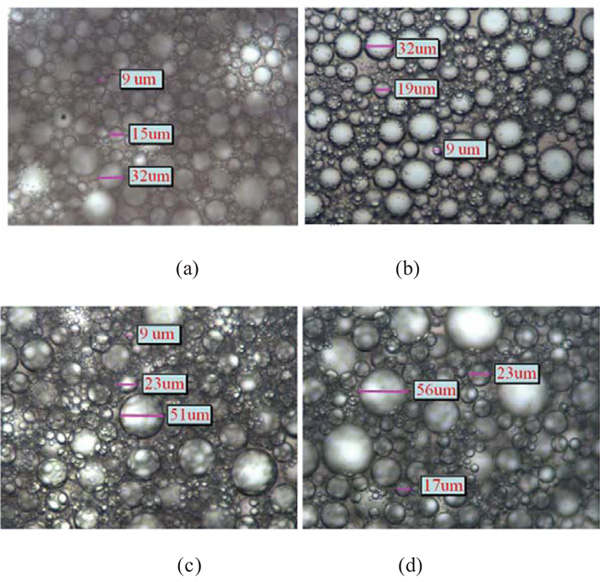
3.2.2. Effect of Fracture Additives
The effects of fracture additives on the particle size of emulsion were shown in Fig. (6). It can be clearly seen that well-distributed droplets were obtained in hydroxypropyl guar and pH adjuster (Na2CO3) emulsion, and the droplets sizes for both emulsions were mainly in 9 μm~32 μm. It is not strange to get a stable emulsion with pH adjuster (Na2CO3). Na2CO3 can react with petroleum acid in crude oil to present a kind of surfactant, which is the main substance for stable emulsion. On contrary, the particle sizes of emulsions prepared from friction reducer (SL-P) and clay stabilizer (FP-2) were much larger. The droplets sizes for each were 9 μm~51 μm and 17 μm~56 μm, respectively. Therefore, hydroxypropyl guar and pH adjuster (Na2CO3) in fracture additives play the main role for the stability of the emulsion.
3.3. Interface behavior of fracture additives
3.3.1. Effects of Fracture Additives on Interfacial Tension
The effects of fracture additives on interfacial tension between crude oil (5% in kerosene) and simulation water were shown in Fig. (7). Each fracture additive was dissolved in simulation water at a concentration of 1000 mg/L in advance. Then the water phase with one of the fracture additives was poured into a rotating capillary tube. Subsequently, a little drop of oil phase (20 μL) was injected into the middle of the same tube. After sealed, the two liquid system rotated with the tube at a speed of 5000 rpm, and the oil drop was stretched. Finally, the diameter of the elongated oil drop was monitored as functions of time until it reached a constant value, and then the interfacial tensions were calculated by the software attached.
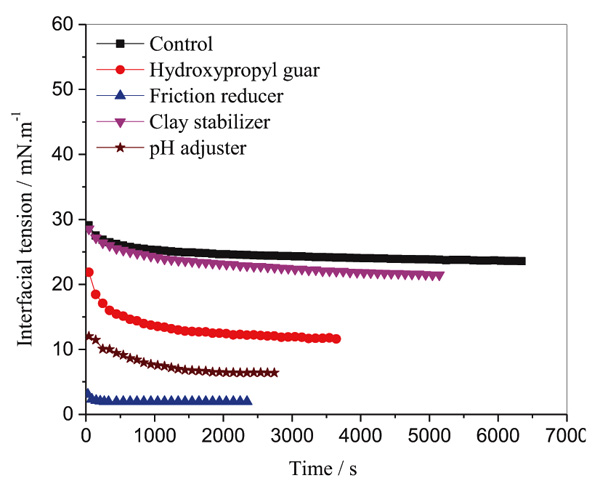 |
Fig. (7). Effects of fracture additives on interfacial tension. |
It can be observed from Fig. (7), the control equilibrium IFT of oil-water was about 24.0 mN/m, and the equilibrium IFT was a little lower when hydroxypropyl guar was added in the water. The lowest interfacial tension was obtained by friction reducer, and the value of equilibrium IFT was 1.9 mN/m. This is because the friction reducer used in Shengli Oil Field is a kind of surfactant named SL-P. The friction reducer reduced the interface energy of oil-water, which is favor for the formation of emulsion. The pH adjuster (Na2CO3) reacts with petroleum acid to present petroleum carbonate, which is a natural surfactant. Therefore, the IFT was decreased to 6.4 mN/m after Na2CO3 was added into the water phase.
3.3.2. Effects of Fracture Additives on Dilational Interfacial Rheology
Dilational rheology are the main characteristics of the equilibrium and dynamic properties of a liquid film and has been proved to be a powerful tool to investigate the properties of adsorption films. Interfacial dilational properties play crucial roles in the formation and brake of crude oil emulsion, and the relationship between dilational data and emulsion stability had been discussed by Zhang et al. [35].
In the present work, the influences of fracture additives on the interfacial film of crude oil and simulated water were studied by the dilational rheological method. The dilational modulus (ε) for fracture additives, such as hydroxypropyl guar, pH adjuster (Na2CO3), friction reducer (SL-P) and clay stabilizer (FP-2) were shown in Fig. (8). As shown, the dilational modulus (ε) increased gradually with time except for friction reducer. For the control film from crude oil and simulated water, the dilational modulus increased from around 6 mN/m to 20 mN/m within 100 min. This is because the active ingredients of oil (such as resins and asphaltenes) gradually adsorbed on the oil-water interface, and then formed a viscoelastic film. As shown in Fig. (8), the curve was not changed too much when clay stabilizer was added into the water phase. The reason is that clay stabilizer is usually ammonium or inorganic salt, such as tetramethyl ammonium chloride, potassium chloride or sodium chloride, which has little influence on the film of emulsion. However, the dilation modulus was increased significantly when hydroxypropyl guar was added into simulated water. The ε value increased from around 10 mN/m to 32 mN/m in 60 min, which indicated that the polymer of hydroxypropyl guar was adsorbed on the oil-water interface, and a viscoelastic film was formed. As referred by Zhang et al. [35] that interfacial dilational rheological data can reflect some aspects of the nature of the film. A direct, reliable and qualitative link may exist between dilational data and the emulsion stability. Generally, coalescence can be described as the formation of a hole in a thin film. Interfacial dilational elasticity are thought to resist the deformation of the film. Therefore, a higher dilational modulus always leads to the formation of a more stable emulsion [36]. From this point of view, hydroxypropyl guar may be the most important stabilizer among the fracture additives for the emulsion. As for pH adjuster, the dilational modulus of which was a little higher than that of control. This is because Na2CO3 reacted with –COOH on resins or asphaltenes in crude oil, and resulted in the formation of hydrophilic –COONa. The saponified resins or asphaltenes adsorbed onto the interface and resulted in the increase of dilational modulus of the film. On the contrary, the dilational modulus was decreased significantly when friction reducer was added into the water phase. This is because that friction reducer is a kind of low molecular weight surfactant. Generally, low molecular weight surfactants and surface active components in crude oil will dramatically reduce the dilational parameters of the interfacial film, which results from the fast diffusion-exchange process of molecules between the bulk and the interface.
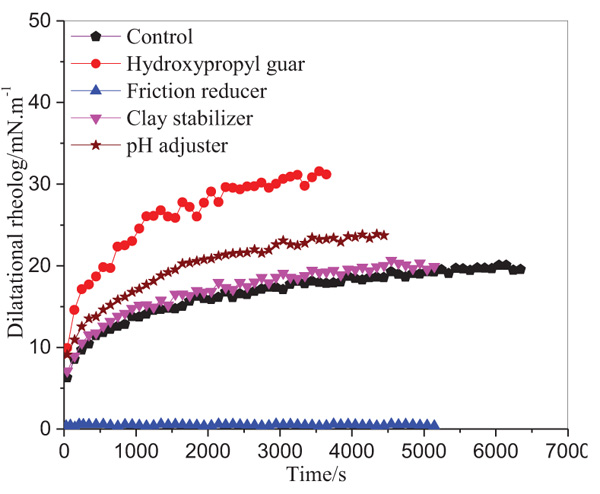 |
Fig. (8). Dilational rheology of fracture additives. Oil phase =5% crude oil; Water phase= fracture additives |
CONCLUSION
The present work studied the stability and stable mechanism of emulsion with fracture fluid. The influences of fracture additives and components of crude oil on the stability of emulsion, size of emulsion particles, interfacial tension and modulus of dilation were investigated systematically. The main results are summarized as follows.
- Resins and asphaltenes among component of crude oil made an important contribution to the emulsion stability.
- Among the fracture additives, hydroxypropyl guar played the key role on the emulsion stability.
- The stabilization mechanism of the emulsion with fracture fluid may be stated as follows: Firstly, as a kind of surfactant, the interface energy of oil-water was reduced by friction reducer, which is in favor for the formation of an emulsion. Secondly, the dilational modulus of crude oil-water film was increased by hydroxypropyl guar significantly. The hydroxypropyl guar adsorbed gradually onto the interface, and generated a viscoelastic film, which resulted in a stable emulsion. The last but not the least, the pH adjuster (Na2CO3) saponified the –COOH on resin and asphaltene. The interaction between crude oil components and fracture additives affected the formation and stability of the emulsion.
- For oil-water separation of the produced liquid with residual fracture liquid, demulsifier with strong demulsifying performance should be applied due to the high stability of the emulsion with fracture additives.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors are thankful for the financial support from the National Science & Technology Major Project (2011ZX05011). The authors are also grateful to Dr. Lu Zhang and Lei Zhang, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences for partial experimental support of these studies.